The hypertrophic scar market has been comprehensively analyzed in this report titled "Hypertrophic Scar Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". A hypertrophic scar refers to a cutaneous condition in which raised, thickened, and wider scars occur around a healing wound due to an abnormal response to an injury or trauma. These scars are characterized by dermal tissue proliferation, prolonged over-deposition of extracellular matrix (ECM) proteins, particularly collagen, as well as persistent inflammation and fibrosis. The condition becomes evident within weeks of the injury and is limited to the area of damaged skin. Individuals suffering from this disorder may experience hard or thickened raised tissue, along with pink to red or purple skin color over the wound site, irritation, itching, tenderness, pain, mild discomfort, etc. In some cases, when a scar develops over the skin of a joint, it might impair the joint's normal movement. The diagnosis of this ailment is mainly based on the patient's medical history and clinical features. The healthcare provider may also perform a physical examination to evaluate the affected area. Additionally, a skin biopsy is required to confirm a diagnosis if the scar continues to change or worsen.
The increasing cases of injury or trauma to the skin, including surgical incisions, burns, cuts, abrasions, etc., are primarily driving the hypertrophic scar market. Furthermore, the rising prevalence of several associated risk factors, such as genetic predisposition, bacterial or fungal infections at the wound site, poor wound care, herpes zoster infection, chicken pox, etc., is also propelling the market growth. In addition to this, the escalating utilization of various medications, including corticosteroid and bleomycin injections, which can soften and flatten the scar and ease symptoms like itching and pain, is creating a positive outlook for the market. Moreover, the inflating usage of tension-releasing surgical techniques, such as Z-plasty and local flap methods, for patients with advanced disease conditions who have failed conservative treatments is also bolstering the market growth. Apart from this, the emerging popularity of laser therapies, since they are less painful and uncomfortable than other scar treatment options like surgical scar revision, is acting as another significant growth-inducing factor. Additionally, the widespread adoption of silicone sheets or ointments to lower scar appearance and improve skin texture in patients is expected to drive the hypertrophic scar market in the coming years.
This report provides an exhaustive analysis of the hypertrophic scar market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for hypertrophic scar and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the hypertrophic scar market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the hypertrophic scar market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the hypertrophic scar market
Competitive Landscape:
This report also provides a detailed analysis of the current hypertrophic scar marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the hypertrophic scar market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the hypertrophic scar market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the hypertrophic scar market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of hypertrophic scar across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hypertrophic scar by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hypertrophic scar by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with hypertrophic scar across the seven major markets?
- What is the size of the hypertrophic scar patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of hypertrophic scar?
- What will be the growth rate of patients across the seven major markets?
Hypertrophic Scar: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for hypertrophic scar drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the hypertrophic scar market?
- What are the key regulatory events related to the hypertrophic scar market?
- What is the structure of clinical trial landscape by status related to the hypertrophic scar market?
- What is the structure of clinical trial landscape by phase related to the hypertrophic scar market?
- What is the structure of clinical trial landscape by route of administration related to the hypertrophic scar market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
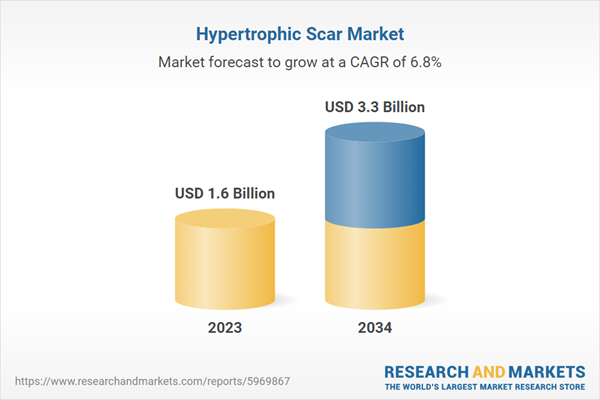
| Estimated Market Value ( USD | $ 1.6 Billion |
| Forecasted Market Value ( USD | $ 3.3 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |