The oral mucositis market has been comprehensively analyzed in this report titled "Oral Mucositis Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Oral mucositis refers to an inflammation and ulceration of the mucosa, the protective mucous membranes that line the mouth, throat, and gastrointestinal tract. It generally occurs as a severely debilitating complication of cancer treatments, particularly radiation, chemotherapy, stem cell transplants, or bone marrow transplants. The most common symptom is discomfort or pain in the mouth or throat, making it difficult to eat, drink, and swallow. Several other symptoms may include redness, swelling, and the development of ulcers or sores in the mouth and throat. In severe cases, the patient may experience bleeding, difficulty speaking, and changes in taste or smell. The diagnosis of the ailment is based on the evaluation of the patient's symptoms, medical history, and physical examination to visualize inflamed tissues. The commonly utilized assessment tools for evaluating the severity of the condition are World Health Organization (WHO) Oral Toxicity Score and the National Cancer Institute Common Toxicity Criteria (NCI-CTC). In some cases, additional testing may be necessary to determine the underlying cause of oral mucositis. This may include blood tests to assess for nutritional deficiencies or infections or imaging procedures to evaluate for damage to the mucous membranes.
The escalating utilization of radiation therapy and chemotherapy for treating cancer, which damage the rapidly dividing cells in the mucous membranes leading to inflammation and ulceration, is primarily driving the oral mucositis market. Moreover, the rising incidences of buccal cavity infections due to various risk factors, such as poor lifestyle regimes, smoking, bad oral hygiene, etc., are also bolstering the market growth. In addition to this, the widespread adoption of potent medications, including anti-inflammatory drugs and topical pain relievers, to reduce tissue swelling and provide symptom relief is acting as another significant growth-inducing factor. Furthermore, multiple key players are making extensive investments in R&D activities to introduce mucoadhesive oral protectants that can form a protective hydrogel coating over the oral mucosa during cancer treatments. This, in turn, is also creating a positive outlook for the market. Additionally, the emerging popularity of cryotherapy for treating the ailment since it can induce local vasoconstriction, which reduces mucosal blood flow and exposure to the cytostatic drug, is further expected to drive the oral mucositis market in the coming years.
This report provides an exhaustive analysis of the oral mucositis market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for oral mucositis and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the oral mucositis market in any manner.
Recent Developments:
In April 2024, OncoZenge AB reported that ongoing stability studies for the improved formulation of BupiZenge have yielded positive results. BupiZenge is a lozenge designed to provide local pain relief to people suffering from oral mucositis.
In January 2024, OraBio announced that Periovance Oral Rinse is generally available for use and purchase. Periovance Oral Rinse is an all-natural liquid anesthetic that provides dental professionals with a simple, fast, and effective alternative for relieving and controlling patient pain both in and out of the office.
In January 2024, Align Technology, Inc. announced the release of the iTero Lumina intraoral scanner. This scanner features a 3X broader field of capture in a 50% smaller and 45% lighter wand, resulting in quicker scanning speed, more accuracy, superior visualization, and a more comfortable scanning experience.
In November 2023, Ensysce Biosciences, Inc. and OncoZenge AB declared that they had signed a letter of intent (LOI) to explore a strategic collaboration to promote the commercialization of BupiZenge in the United States. The proposed agreement aims to capitalize on both firms' expertise and resources to accelerate the path to market in the United States for this novel medication that addresses significant unmet needs in analgesia and oncology.
Key Highlights:
Oral mucositis develops in around 40% of cancer treatment patients.
The incidences of mild, moderate, and severe oral mucositis were found worldwide to be 16.8%, 34.5%, and 26.4%, respectively.
The incidence of oral mucositis is higher in patients who undergo continuous infusion therapy for breast and colon cancer, as well as those who receive adjuvant treatment for head and neck cancers.
Radiotherapy-induced oral mucositis is the most common and painful radiation complication, with a frequency ranging from 85.0% to 100.0% in patients with head and neck cancer during treatment.
Oral mucositis may be more common among younger people.
Drugs:
Kepivance refers to a recombinant human keratinocyte growth factor that acts at the cellular level to safeguard patients with hematologic malignancies receiving high-dose chemotherapy and/or radiation followed by a bone marrow transplant from severe oral mucositis. Kepivance reduces the occurrence and duration of severe oral mucositis in these patients by preserving the epithelial cells that line the throat and mouth from damage caused by chemotherapy and radiation, as well as stimulating the growth and development of new epithelial cells to form the mucosal barrier.
Clonidine HCl is a new mucobuccal tablet (MBT) that allows for extended local administration of clonidine to areas of oral mucosal radiation damage in patients with oropharyngeal carcinoma (OPC). The pill is self-administered once daily at home by the patient inserting it beneath the upper lip, where it sticks to the gums and degrades over several hours, continually releasing clonidine into the saliva. It antagonizes the alpha-2 adrenergic receptor on macrophages, thereby reducing the production of harmful cytokines produced by macrophages in response to radiation.
SGX942 is an intravenous form of the innate defense regulator, dusquetide, used to treat severe oral mucositis. It is a quick 4-minute infusion given twice a week during chemotherapy and/or radiation treatment that is being studied to lessen the duration and severity of severe oral mucositis.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the oral mucositis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the oral mucositis market
Competitive Landscape:
This report also provides a detailed analysis of the current oral mucositis marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the oral mucositis market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the oral mucositis market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the oral mucositis market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of oral mucositis across the seven major markets?
- What is the number of prevalent cases (2018-2034) of oral mucositis by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of oral mucositis by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with oral mucositis across the seven major markets?
- What is the size of the oral mucositis patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of oral mucositis?
- What will be the growth rate of patients across the seven major markets?
Oral Mucositis: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for oral mucositis drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the oral mucositis market?
- What are the key regulatory events related to the oral mucositis market?
- What is the structure of clinical trial landscape by status related to the oral mucositis market?
- What is the structure of clinical trial landscape by phase related to the oral mucositis market?
- What is the structure of clinical trial landscape by route of administration related to the oral mucositis market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 138 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
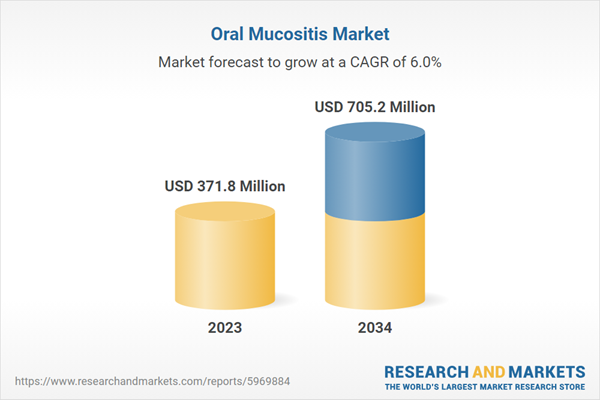
| Estimated Market Value ( USD | $ 371.8 Million |
| Forecasted Market Value ( USD | $ 705.2 Million |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |