The Angelman syndrome market has been comprehensively analyzed in this report titled "Angelman Syndrome Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Angelman syndrome is a complex genetic condition that affects the nervous system. The disorder is mainly caused by mutations in the UBE3A gene in chromosome region 15, which is responsible for producing proteins involved in the development and maintenance of various organs in the body. Angelman syndrome is characterized by intellectual disability or severe developmental delay, speech impairment, tremulousness of the limbs and/or gait ataxia, microcephaly (small head size), recurrent seizures, etc. Some of the common symptoms associated with this ailment include trouble sleeping, hand-flapping movements, unique behavior with frequent smiling, laughing, excitability, etc. The diagnosis of Angelman syndrome typically involves a combination of clinical evaluation, medical history, and genetic testing. Physicians may perform a physical exam to look for characteristic features of the condition, such as hypopigmented skin and eyes and abnormal side-to-side spine curvature. Blood and gene tests are used to detect genetic mutations associated with the disease. Additionally, radiographic examinations, including brain MRI and CT scans, can confirm the diagnosis of the ailment. In some cases, various DNA methylation studies are also utilized to evaluate the chromosome defects that cause the disorder.
The increasing cases of genetic disorders and the rising unmet need for effective treatments to address the underlying congenital defect and improve patient outcomes are primarily driving the Angelman syndrome market. In addition to this, the widespread utilization of novel medications, including anticonvulsants, mild laxatives, sedatives, etc., to reduce the symptoms of the condition is creating a positive outlook for the market. Moreover, the escalating adoption of electroencephalogram (EEG), which measures abnormal brain activity with a characteristic pattern of large-amplitude slow-spike waves to diagnose seizures or other neurological problems associated with Angelman syndrome, is further bolstering the market growth. Apart from this, the emerging popularity of CRISPR gene editing technology, which can override the DNA mutations underlying Angelman syndrome, is acting as another significant growth-inducing factor. Furthermore, several key players are making extensive investments in achieving a better understanding of the natural history of the ailment, including the course of disease progression and factors that influence outcomes, for creating novel treatments. This, in turn, is also augmenting the market growth. Additionally, the ongoing development of antisense oligonucleotide therapeutics for providing targeted and long-lasting effects, with the possibility of disease modification rather than just symptom relief, is expected to drive the Angelman syndrome market during the forecast period.
This report provides an exhaustive analysis of the Angelman syndrome market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for Angelman syndrome and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the Angelman syndrome market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Angelman syndrome market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Angelman syndrome market
Competitive Landscape:
This report also provides a detailed analysis of the current Angelman syndrome marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the Angelman syndrome market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the Angelman syndrome market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the Angelman syndrome market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of Angelman syndrome across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Angelman syndrome by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Angelman syndrome by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with Angelman syndrome across the seven major markets?
- What is the size of the Angelman syndrome patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of Angelman syndrome?
- What will be the growth rate of patients across the seven major markets?
Angelman Syndrome: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Angelman syndrome drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Angelman syndrome market?
- What are the key regulatory events related to the Angelman syndrome market?
- What is the structure of clinical trial landscape by status related to the Angelman syndrome market?
- What is the structure of clinical trial landscape by phase related to the Angelman syndrome market?
- What is the structure of clinical trial landscape by route of administration related to the Angelman syndrome market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 128 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
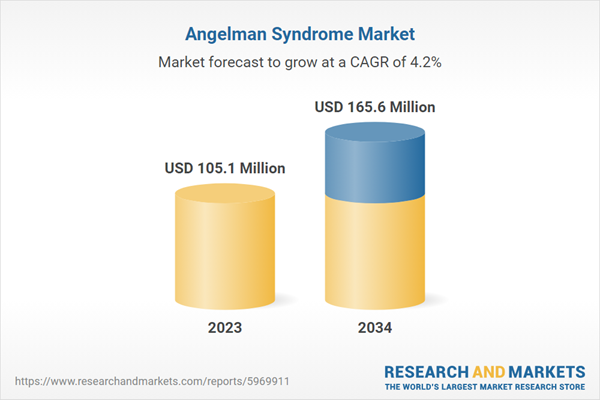
| Estimated Market Value ( USD | $ 105.1 Million |
| Forecasted Market Value ( USD | $ 165.6 Million |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | Global |