The allergic rhinitis market has been comprehensively analyzed in this report titled "Allergic Rhinitis Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Allergic rhinitis, or hay fever, is a type of nasal inflammation caused by the immune system overreacting to allergens in the air. This reaction causes the mucous membrane of the nose to release a variety of chemicals, causing the nose to swell and produce an excessive amount of mucus. Some of the common symptoms are sneezing, nasal congestion, clear rhinorrhea, nasal pruritus, postnasal drip, etc. Individuals suffering from allergic rhinitis may also experience conjunctival swelling and erythema, lower eyelid venous stasis, eyelid swelling, middle ear effusion, swollen nasal turbinates, etc. The diagnosis of allergic rhinitis typically involves a combination of patient history, physical examination, and allergy testing. Medical history and physical examination can provide information on the duration and frequency of symptoms, as well as any associated conditions, such as asthma. Allergy testing may involve skin prick tests or blood tests to identify specific allergens triggering the allergic reaction. Various additional investigations, including nasal endoscopy, may be performed to rule out other potential causes of symptoms.
The increasing prevalence of allergic reactions to various allergens, such as pollen, pet dander, dust mites, mold, etc., is primarily driving the allergic rhinitis market. Moreover, the escalating utilization of topical nasal corticosteroids, since they aid in reducing inflammation in the nasal passages and alleviate symptoms, including congestion, sneezing, runny nose, etc., is also bolstering the market growth. Besides this, the widespread adoption of rhinomanometry, which measures the resistance of airflow through the nasal passages to identify any blockages or narrowing that may be causing the ailment, is acting as another significant growth-inducing factor. Additionally, the ongoing development of advanced drug delivery systems, such as nasal sprays and inhalers, is improving the efficacy and convenience of treatment for allergic rhinitis patients, thereby propelling the market growth. Furthermore, the rising application of the basophil activation test, which reduces the need for in-vivo procedures and specifically detects all the characteristics of IgE and allergens in the diagnosis of allergic disease, is positively influencing the market. Apart from this, the emerging popularity of sublingual allergen immunotherapy owing to its several associated benefits, including ease of self-administration and reduced risk for systemic reactions, is expected to drive the allergic rhinitis market during the forecast period.
This report provides an exhaustive analysis of the allergic rhinitis market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for allergic rhinitis and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the allergic rhinitis market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the allergic rhinitis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the allergic rhinitis market
Competitive Landscape:
This report also provides a detailed analysis of the current allergic rhinitis marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the allergic rhinitis market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the allergic rhinitis market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the allergic rhinitis market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of allergic rhinitis across the seven major markets?
- What is the number of prevalent cases (2018-2034) of allergic rhinitis by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of allergic rhinitis by gender across the seven major markets?
- What is the number of prevalent cases (2018-2034) of allergic rhinitis by type across the seven major markets?
- How many patients are diagnosed (2018-2034) with allergic rhinitis across the seven major markets?
- What is the size of the allergic rhinitis patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of allergic rhinitis?
- What will be the growth rate of patients across the seven major markets?
Allergic Rhinitis: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for allergic rhinitis drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the allergic rhinitis market?
- What are the key regulatory events related to the allergic rhinitis market?
- What is the structure of clinical trial landscape by status related to the allergic rhinitis market?
- What is the structure of clinical trial landscape by phase related to the allergic rhinitis market?
- What is the structure of clinical trial landscape by route of administration related to the allergic rhinitis market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 130 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
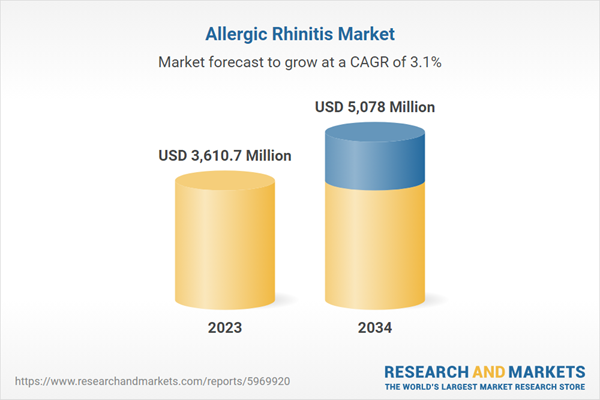
| Estimated Market Value ( USD | $ 3610.7 Million |
| Forecasted Market Value ( USD | $ 5078 Million |
| Compound Annual Growth Rate | 3.1% |
| Regions Covered | Global |