The synucleinopathies market has been comprehensively analyzed in this report titled "Synucleinopathies Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Synucleinopathies refer to a group of neurodegenerative disorders characterized by the abnormal buildup of a protein called alpha-synuclein in certain regions of the brain. This protein misfolds and aggregates into clumps or fibrils, forming insoluble deposits known as Lewy bodies. The common symptoms of these conditions include rest tremors, rigidity, slowness of movement, postural instability, cognitive decline, recurrent visual hallucinations, fluctuations in attention and alertness, autonomic dysfunction, sleep disturbances, balance problems, coordination difficulties, etc. In some cases, individuals suffering from synucleinopathies might also experience a drop in blood pressure upon standing up, which can lead to dizziness, lightheadedness, or even fainting. The diagnosis of these ailments is typically made by taking a detailed medical history and conducting a thorough physical examination. Additionally, a dopamine transporter (DAT) imaging test using positron emission tomography scans or single-photon emission computed tomography scans is utilized to assess the integrity of dopamine-producing cells in the brain. The healthcare provider may further recommend lumbar puncture techniques to measure levels of specific biomarkers, such as alpha-synuclein and total tau, in patients.
The escalating cases of variations in certain genes leading to abnormal production of alpha-synuclein protein, which can promote its aggregation and accumulation in nerve cells, are primarily driving the synucleinopathies market. In addition to this, the growing geriatric population, who are prone to neuronal damage due to increased oxidative stress and chronic inflammation within the brain, is creating a positive outlook for the market. Moreover, the widespread adoption of orthotic devices, such as ankle-foot orthoses (AFOs), since they can provide support to the lower limbs and maintain better balance while walking or standing, thereby reducing the risk of falls and improving overall mobility, is further bolstering the market growth. Apart from this, the inflating application of monoamine oxidase-B inhibitors, like rasagiline and selegiline, to inhibit the breakdown of dopamine in the brain and increase its availability is acting as another significant growth-inducing factor. Additionally, the emerging popularity of deep brain stimulation procedures, which involve implanting electrodes into specific areas of the brain and delivering electrical impulses to modulate abnormal neural activity, is expected to drive the synucleinopathies market during the forecast period.
This report provides an exhaustive analysis of the synucleinopathies market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for synucleinopathies and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the synucleinopathies market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the synucleinopathies market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the synucleinopathies market
Competitive Landscape:
This report also provides a detailed analysis of the current synucleinopathies marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the synucleinopathies market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the synucleinopathies market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the synucleinopathies market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of synucleinopathies across the seven major markets?
- What is the number of prevalent cases (2018-2034) of synucleinopathies by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of synucleinopathies by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with synucleinopathies across the seven major markets?
- What is the size of the synucleinopathies patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of synucleinopathies?
- What will be the growth rate of patients across the seven major markets?
Synucleinopathies: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for synucleinopathies drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the synucleinopathies market?
- What are the key regulatory events related to the synucleinopathies market?
- What is the structure of clinical trial landscape by status related to the synucleinopathies market?
- What is the structure of clinical trial landscape by phase related to the synucleinopathies market?
- What is the structure of clinical trial landscape by route of administration related to the synucleinopathies market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
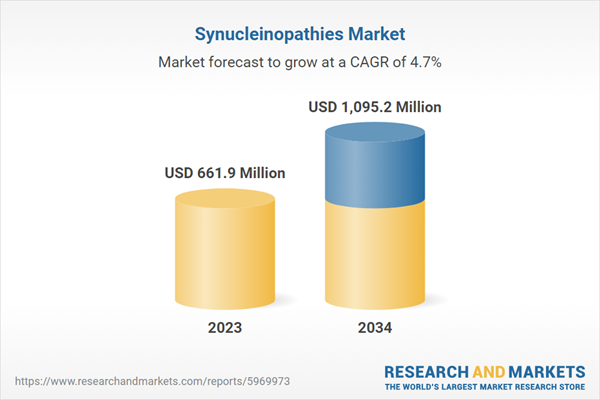
| Estimated Market Value ( USD | $ 661.9 Million |
| Forecasted Market Value ( USD | $ 1095.2 Million |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |