The TDP-43 proteinopathies market has been comprehensively analyzed in this report titled "TDP-43 Proteinopathies Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". TDP-43 proteinopathies are a group of neurodegenerative disorders characterized by the irregular accumulation of a protein called TAR DNA-binding protein 43 (TDP-43) in the brain and spinal cord. This protein is mainly essential for RNA processing and regulation, but in these diseases, it becomes abnormally modified and forms aggregates within neurons. The common symptoms associated with TDP-43 proteinopathies include changes in thinking, memory, and behavior, muscle weakness, atrophy, twitching, difficulties with movement control, language, and speech problems, apathy, disinhibition, social withdrawal, etc. Individuals suffering from these ailments may also experience trouble performing daily activities, such as dressing, eating, bathing, using tools or objects, etc. The diagnosis of TDP-43 proteinopathies typically involves a combination of clinical evaluation, medical history review, and pathological examination. Various neuroimaging techniques, like magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, may be performed to assess brain structure and function. These studies can help to identify patterns of atrophy or abnormal metabolic activity that are associated with the conditions. Furthermore, the healthcare provider may recommend an analysis of cerebrospinal fluid to measure protein levels and confirm a diagnosis.
The increasing cases of genetic mutations, which can lead to abnormal aggregation and accumulation of proteins within the body, are primarily driving the TDP-43 proteinopathies market. Besides this, the rising geriatric population, who are susceptible to age-related cellular and molecular changes, such as impaired protein homeostasis and enhanced oxidative stress, is creating a positive outlook for the market. Moreover, the widespread adoption of various medications, including antipsychotics, antidepressants, sleep aids, etc., which work by targeting specific neurotransmitter systems in the brain to alleviate disease symptoms and restore cognitive abilities in patients, is further bolstering the market growth. Apart from this, the inflating application of assistive devices, such as canes, walkers, wheelchairs, etc., since they help to reduce mobility impairments, maintain independence, and improve daily functioning, is acting as another significant growth-inducing factor. Additionally, the emerging popularity of small molecule therapeutics that can specifically target the pathological processes associated with the condition, thereby decreasing the formation of abnormal protein aggregates, is expected to drive the TDP-43 proteinopathies market during the forecast period.
This report provides an exhaustive analysis of the TDP-43 proteinopathies market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for TDP-43 proteinopathies and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the TDP-43 proteinopathies market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the TDP-43 proteinopathies market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the TDP-43 proteinopathies market
Competitive Landscape:
This report also provides a detailed analysis of the current TDP-43 proteinopathies marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the TDP-43 proteinopathies market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the TDP-43 proteinopathies market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the TDP-43 proteinopathies market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of TDP-43 proteinopathies across the seven major markets?
- What is the number of prevalent cases (2018-2034) of TDP-43 proteinopathies by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of TDP-43 proteinopathies by gender across the seven major markets?
- What is the number of prevalent cases (2018-2034) of TDP-43 proteinopathies by type across the seven major markets?
- How many patients are diagnosed (2018-2034) with TDP-43 proteinopathies across the seven major markets?
- What is the size of the TDP-43 proteinopathies patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of TDP-43 proteinopathies?
- What will be the growth rate of patients across the seven major markets?
TDP-43 Proteinopathies: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for TDP-43 proteinopathies drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the TDP-43 proteinopathies market?
- What are the key regulatory events related to the TDP-43 proteinopathies market?
- What is the structure of clinical trial landscape by status related to the TDP-43 proteinopathies market?
- What is the structure of clinical trial landscape by phase related to the TDP-43 proteinopathies market?
- What is the structure of clinical trial landscape by route of administration related to the TDP-43 proteinopathies market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
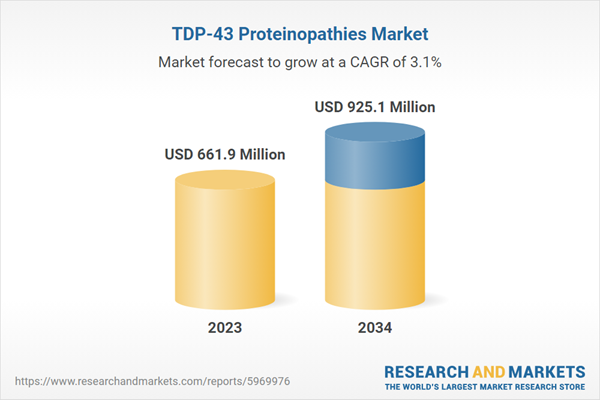
| Estimated Market Value ( USD | $ 661.9 Million |
| Forecasted Market Value ( USD | $ 925.1 Million |
| Compound Annual Growth Rate | 3.1% |
| Regions Covered | Global |