The hepatitis A market has been comprehensively analyzed in this report titled "Hepatitis A Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Hepatitis A refers to a viral infection that occurs in the liver. It is triggered by the hepatitis A virus, which is usually transmitted through the consumption of contaminated water or food or via close contact with an infected person. The common symptoms of this illness include fatigue, yellowing of the skin and eyes, a decrease in appetite, unintentional weight loss, nausea, vomiting, abdominal discomfort, dark urine, pale-colored stools, muscle and joint pain, fever, intense itching, diarrhea, etc. The diagnosis of hepatitis A typically involves a combination of medical history, physical examination, laboratory procedures, and sometimes imaging studies. The healthcare provider may perform a blood work-up to detect the presence of antibodies generated by the immune system in response to the illness. Various liver function tests, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), are also required to measure the levels of proteins and enzymes in the blood. In addition to this, a stool sample examination is utilized to check for the presence of a virus in the feces and confirm a diagnosis among patients.
The increasing incidences of poor hygiene practices and inadequate sanitation facilities, which can enhance the likelihood of virus transmission, are primarily driving the hepatitis A market. In addition to this, the rising prevalence of intravenous drug use that involves the sharing of needles, syringes, or other drug paraphernalia among individuals is also bolstering the market growth. Furthermore, the widespread adoption of effective medications, such as analgesics, antiemetics, pain relievers, etc., to alleviate symptoms of the ailment and improve the quality of life is acting as another significant growth-inducing factor. Additionally, the escalating utilization of hepatitis A vaccination, since it stimulates the immune system to produce antibodies that fight off the virus, thereby preventing infection, is also creating a positive outlook for the market. Moreover, the emerging popularity of molecular diagnostic techniques, such as polymerase chain reaction, which can be used to detect and amplify the genetic material (RNA) of the virus even in the early stages of infection when antibody levels may be low, is expected to drive the hepatitis A market in the coming years.
This report provides an exhaustive analysis of the hepatitis A market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for hepatitis A and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the hepatitis A market in any manner.
Recent Developments:
In March 2024, European health authorities reported the presence of hepatitis A in Moroccan strawberries that were transported to Spain. The contamination has been identified as exceeding the virus's maximum allowable limit, representing a significant public health risk.
In January 2024, Indian Immunologicals (IIL) launched the indigenously developed hepatitis A vaccine called Havisure. It is a two-dose vaccine; the initial dosage will be administered to children over the age of 12 months, and the second dose will be delivered at least six months later. Havisure is indicated for children as part of their routine vaccination regimen, as well as people who may have been exposed to hepatitis A or plan to visit high-prevalence areas.
Key Highlights:
According to the WHO, the majority of children (90%) get infected with the hepatitis A virus before the age of ten years, usually without symptoms.
Infection rates are low in high-income countries compared to low - and middle-income countries with poor sanitary and hygienic practices.
The estimated incidence of new cases of hepatitis A is approximately 1.5 million, along with 11,000 deaths a year worldwide.
In the United States, the hepatitis A incidence has reduced by 90% to as low as 1.2 cases per 100,000 population.
In accordance with the Centers for Disease Control and Prevention (CDC), the mortality rate of hepatitis A virus infection was 6 times higher among males (0.06 deaths per 100,000 population) than the rate among females (0.01 deaths per 100,000 population).
Drugs:
Twinrix is a vaccine manufactured by GlaxoSmithKline Biologicals. It is authorized by the United States Food and Drug Administration (FDA) to prevent hepatitis A virus infection in individuals aged 18 and older. The vaccine is injected intramuscularly in the deltoid area on a regimen of three different doses at 0, 1, and 6 months.
BR 8003 is an inactivated hepatitis A virus vaccine manufactured by Boryung Pharmaceutical for the prevention of hepatitis A infection. The therapeutic candidate stimulates the body's defense mechanism to develop antibodies against the virus, thereby providing immunity to the disease.
Hepatitis A vaccine is developed by Biological E Ltd. to protect against the hepatitis A virus infection. It is typically administered as an injection in a two-dose schedule, with the second dose given six to twelve months after the first. The vaccine can generate antibodies against the pathogen to provide immunity and prevent the contraction of the disease.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the hepatitis A market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the hepatitis A market
Competitive Landscape:
This report also provides a detailed analysis of the current hepatitis A marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the hepatitis A market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the hepatitis A market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the hepatitis A market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of hepatitis A across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hepatitis A by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hepatitis A by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with hepatitis A across the seven major markets?
- What is the size of the hepatitis A patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of hepatitis A?
- What will be the growth rate of patients across the seven major markets?
Hepatitis A: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for hepatitis A drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the hepatitis A market?
- What are the key regulatory events related to the hepatitis A market?
- What is the structure of clinical trial landscape by status related to the hepatitis A market?
- What is the structure of clinical trial landscape by phase related to the hepatitis A market?
- What is the structure of clinical trial landscape by route of administration related to the hepatitis A market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 135 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
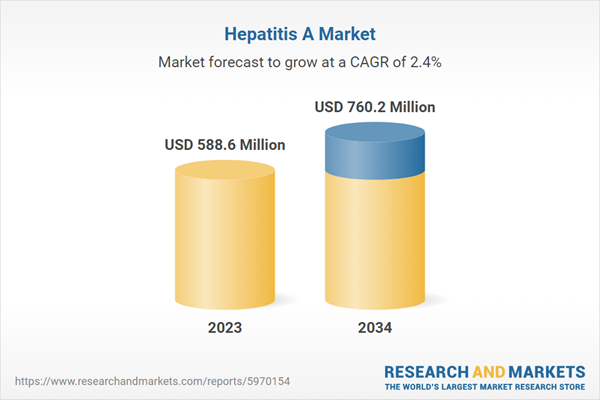
| Estimated Market Value ( USD | $ 588.6 Million |
| Forecasted Market Value ( USD | $ 760.2 Million |
| Compound Annual Growth Rate | 2.4% |
| Regions Covered | Global |