The desmoid tumors market has been comprehensively analyzed in this report titled "Desmoid Tumors Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Desmoid tumors, also called aggressive fibromatosis, refer to rare non-cancerous growths that develop in the connective tissues of the body, such as tendons, muscles, and ligaments. Despite being benign, these cancerous cells can be locally invasive and cause significant morbidity due to their aggressive nature. The symptoms of these tumors can vary depending on their location and size. Common signs of the ailment may include the presence of a palpable mass or swelling in the affected region, a limited range of motion, and pain. In some cases, these tumors can impinge on nearby structures, leading to additional symptoms like bowel obstruction or compression of nerves. Diagnosing desmoid tumors can be challenging as they often mimic other benign and malignant conditions. The process typically involves a thorough physical examination, a medical history review, and imaging tests, such as MRI or CT scans, to visualize the tumor's location and extent. Additionally, a biopsy is necessary to confirm the diagnosis and differentiate the disease from more aggressive malignancies.
The increasing cases of genetic predisposition and mutations in specific genes, leading to the abnormal proliferation of fibroblasts, the cells responsible for connective tissue development, are primarily driving the desmoid tumors market. In addition to this, the inflating utilization of efficient treatment methods, such as nonsteroidal anti-inflammatory drugs, hormonal therapy, chemotherapy, etc., to manage and control the proliferation of cancerous cells is also creating a positive outlook for the market. Moreover, the widespread adoption of physical rehabilitation techniques, which aid in restoring bodily function and muscle strength after surgical intervention, is further bolstering the market growth. Apart from this, the rising usage of radiotherapy, since it aims directly at the tumor site to prevent the recurrence of cancerous cells while sparing the surrounding healthy tissues, is acting as another significant growth-inducing factor. Additionally, the emerging popularity of cryoablation procedures, on account of their several benefits, such as shorter recovery times and decreased risk of side effects, is also augmenting the market growth. Furthermore, the escalating application of gene therapy, that offers the potential to introduce corrected genetic material to replace or adjust the mutated genes causing the illness, is expected to drive the desmoid tumors market during the forecast period.
This report provides an exhaustive analysis of the desmoid tumors market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for desmoid tumors and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the desmoid tumors market in any manner.
Recent Developments:
In February 2024, Ayala Pharmaceuticals, Inc. declared that the Phase 3 RINGSIDE project, which is assessing AL102 in desmoid tumors, has finished enrolling patients. The company enrolled 156 patients for the trial.
In November 2023, SpringWorks Therapeutics, Inc. stated that the United States Food and Drug Administration (FDA) approved OGSIVEO (nirogacestat), an oral gamma-secretase inhibitor, for the management of adult patients with progressive desmoid tumors requiring systemic treatment. Nirogacestat has previously been designated by the FDA as a breakthrough therapy, fast-track, orphan medication for the treatment of desmoid tumors.
Key Highlights:
Desmoid tumors are uncommon tumors, with a reported frequency of 2-4 per million people, accounting for 0.03% of all neoplasms.
Every year, between 900 and 1,500 people in the United States are diagnosed with a desmoid tumor.
According to epidemiological research, the majority of desmoid tumor cases occur between the ages of 20 and 44, with females exceeding males by 2.2-3.9 times.
In patients with desmoid tumors, familial adenomatous polyposis can be diagnosed in up to 5% of patients.
Spontaneous regressions of the condition are noted in as many as 20%-30% of cases.
Drugs:
Nirogacestat is an oral, selective, small-molecule gamma-secretase inhibitor (GSI) licensed in the United States for the treatment of individuals with advanced desmoid tumors who require systemic therapy. Gamma secretase cleaves numerous transmembrane proteins, including Notch, which play a vital role in activating pathways that contribute to the formation of desmoid cancers.
AL102 is an experimental small molecule gamma-secretase inhibitor that is being tested in the randomized Phase 3 RINGSIDE international study for the treatment of desmoid tumors. AL102 is a potential once-daily oral therapy for desmoid tumors.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the desmoid tumors market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the desmoid tumors market
Competitive Landscape:
This report also provides a detailed analysis of the current desmoid tumors marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the desmoid tumors market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the desmoid tumors market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the desmoid tumors market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of desmoid tumors across the seven major markets?
- What is the number of prevalent cases (2018-2034) of desmoid tumors by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of desmoid tumors by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with desmoid tumors across the seven major markets?
- What is the size of the desmoid tumors patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of desmoid tumors?
- What will be the growth rate of patients across the seven major markets?
Desmoid Tumors: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for desmoid tumors drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the desmoid tumors market?
- What are the key regulatory events related to the desmoid tumors market?
- What is the structure of clinical trial landscape by status related to the desmoid tumors market?
- What is the structure of clinical trial landscape by phase related to the desmoid tumors market?
- What is the structure of clinical trial landscape by route of administration related to the desmoid tumors market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
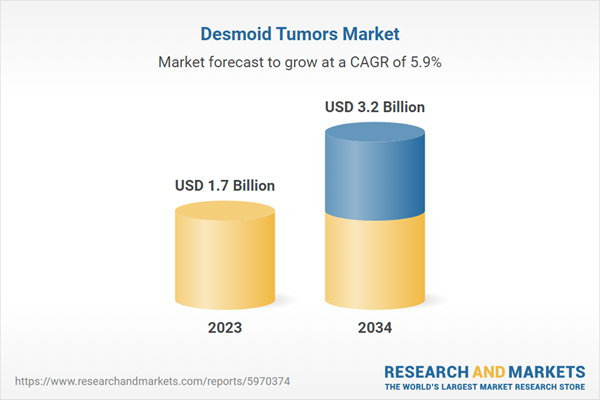
| Estimated Market Value ( USD | $ 1.7 Billion |
| Forecasted Market Value ( USD | $ 3.2 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |