The Asherman's syndrome market has been comprehensively analyzed in this report titled "Asherman's Syndrome Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Asherman's syndrome, also known as intrauterine adhesions or uterine synechiae, is a rare gynecological condition characterized by the development of scar tissue inside the uterus. This scarring can lead to the walls of the uterus sticking together and may result in various reproductive issues. Some of the common symptoms associated with the condition include menstrual irregularities, such as reduced menstrual flow or absence of menstruation (amenorrhea), recurrent pregnancy loss, infertility, etc. The presence of adhesions can obstruct the uterine cavity, affecting the implantation of embryos and lowering the chances of successful pregnancies. Some patients may also experience pelvic pain. The diagnosis of Asherman's syndrome involves a comprehensive evaluation by a healthcare professional with expertise in reproductive health and gynecology. The gold standard for diagnosis is hysteroscopy, a minimally invasive procedure in which a thin, lighted camera (hysteroscope) is inserted through the vagina and cervix to visualize the uterine cavity directly. Additionally, several imaging modalities, such as transvaginal ultrasonography, saline infusion sonohysterography (SIS), hysterosalpingography (HSG), magnetic resonance imaging (MRI), etc., may be performed to visualize the uterine cavity and identify any intrauterine adhesions or abnormalities.
The increasing utilization of dilation and curettage procedures, which can result in injury to the endometrial lining, thereby leading to scar tissue formation and adhesions, is primarily driving the Asherman's syndrome market. In addition to this, the inflating incidences of numerous associated risk factors, including uterine infections like postpartum infections or pelvic inflammatory disease (PID), myomectomy, uterine rupture during childbirth, chronic endometritis, cesarean section deliveries, etc., are also creating a positive outlook for the market. Moreover, the widespread adoption of hormonal therapy to promote endometrial regeneration and reduce the risk of adhesion recurrence is further bolstering the market growth. Apart from this, the escalating application of hysteroscopic adhesiolysis, since it aids in restoring normal uterine anatomy, leading to improved menstrual function and increased chances of conception, is acting as another significant growth-inducing factor. Additionally, the ongoing advancements in the field of diagnostics, such as the introduction of transvaginal ultrasonography that allows visualization of the uterine cavity and the detection of intrauterine adhesions or irregularities in the endometrial lining, are expected to drive the Asherman's syndrome market during the forecast period.
This report provides an exhaustive analysis of the Asherman's syndrome market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for Asherman's syndrome and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the Asherman's syndrome market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Asherman's syndrome market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Asherman's syndrome market
Competitive Landscape:
This report also provides a detailed analysis of the current Asherman's syndrome marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the Asherman's syndrome market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the Asherman's syndrome market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the Asherman's syndrome market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of Asherman's syndrome across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Asherman's syndrome by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Asherman's syndrome by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with Asherman's syndrome across the seven major markets?
- What is the size of the Asherman's syndrome patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of Asherman's syndrome?
- What will be the growth rate of patients across the seven major markets?
Asherman's Syndrome: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Asherman's syndrome drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Asherman's syndrome market?
- What are the key regulatory events related to the Asherman's syndrome market?
- What is the structure of clinical trial landscape by status related to the Asherman's syndrome market?
- What is the structure of clinical trial landscape by phase related to the Asherman's syndrome market?
- What is the structure of clinical trial landscape by route of administration related to the Asherman's syndrome market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 139 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
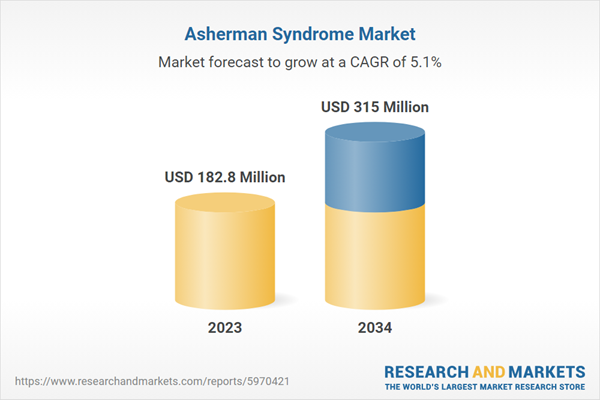
| Estimated Market Value ( USD | $ 182.8 Million |
| Forecasted Market Value ( USD | $ 315 Million |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |