Despite these positive drivers, the market faces hurdles such as complexity of dural repair. However, advances in biomaterials, tissue engineering, and adhesive technologies along with expanding global market for neurosurgical procedures, present lucrative opportunities for the expansion of dural adhesive agent for surgical demand, suggesting a vibrant future for this market as it navigates through challenges towards budgetary constraints and cost pressures as it perceived as more expensive than conventional surgical techniques.
North America experiences a significant burden of neurological disorders, including traumatic brain injuries, brain tumors, and spinal cord injuries. The growing prevalence of these conditions drives the demand for neurosurgical interventions, including the use of dural adhesive agents for repairing dural defects. North America, particularly the U.S. and Canada, has advanced healthcare infrastructure and access to specialized medical services. Brain surgery is relatively common in North America due to the presence of numerous neurosurgical centers and a high prevalence of neurological disorders such as brain tumors, epilepsy, and cerebrovascular diseases.
The trend towards minimally invasive surgery (MIS) techniques in neurosurgery has influenced the demand for dural adhesive agents that are compatible with these approaches. Manufacturers are developing adhesives specifically designed for use in minimally invasive procedures, offering formulations that facilitate precise application and effective dural closure through small incisions or access ports. For instance, in March 2024, a team of researchers, spearheaded by Wyss Institute Founding Core Faculty members, showcased the superior performance of their """"Dural Tough Adhesive"""" (DTA) compared to existing surgical sealants. This assertion was substantiated through testing utilizing in vivo animal models and human-derived tissues ex vivo. These findings have been documented and published in Science Translational Medicine. Hydrogels are emerging as promising materials for dural adhesive applications due to their biocompatibility, flexibility, and adhesive properties. Hydrogel-based dural adhesives can conform to irregular tissue surfaces, providing effective sealing and dural repair. Researchers are exploring novel hydrogel formulations with tunable properties for enhanced performance in neurosurgical applications.
Market Segmentation:
Segmentation 1: by Application
- Cranial Surgery
- Spinal Surgery
Segmentation 2: by Type
- Polyethylene glycol
- Others (Fibrin Glue)
Segmentation 3: by Form
- Sealant Glue
- Sealant Film
Segmentation 4: by Region
- North America
- Europe
- Asia-Pacific
- Rest-of-the-World
How can this report add value to an organization?
Product/Innovation Strategy: The global dural adhesive agent for surgical market has been extensively segmented based on various categories, such as application, type, and form. This can help readers get a clear overview of which segments account for the largest share and which ones are well-positioned to grow in the coming years.Competitive Strategy: A detailed competitive benchmarking of the players operating in the global dural adhesive agent for surgical market has been done to help the reader understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analysing company coverage, product portfolio, and market penetration.Some of the prominent companies in this market are:
- Integra LifeSciences Corporation
- Covidien (Medtronic)
- Baxter
- Johnson & Johnson MedTech
Key Questions Answered in this Report:
- What are the main factors driving the demand for dural adhesive agent for surgical market?
- What are the major patents filed by the companies active in the dural adhesive agent for surgical market?
- Who are the key players in the dural adhesive agent for surgical market, and what are their respective market shares?
- What partnerships or collaborations are prominent among stakeholders in the dural adhesive agent for surgical market?
- What are the strategies adopted by the key companies to gain a competitive edge in dural adhesive agent for surgical market?
- What is the futuristic outlook for the dural adhesive agent for surgical market in terms of growth potential?
- What is the current estimation of the dural adhesive agent for surgical market, and what growth trajectory is projected from 2024 to 2034?
- Which application, and product segment is expected to lead the market over the forecast period (2024-2034)?
- Which regions demonstrate the highest adoption rates for dural adhesive agent for surgical market, and what factors contribute to their leadership?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Medprin
- Integra LifeSciences Corporation
- Pramand, LLC (CraniSeal)
- Stryker
- DuraStat, Inc.
- Covidien (Medtronic)
- Becton
- Regenity
- Baxter
- Johnson & Johnson MedTech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | May 2024 |
| Forecast Period | 2024 - 2034 |
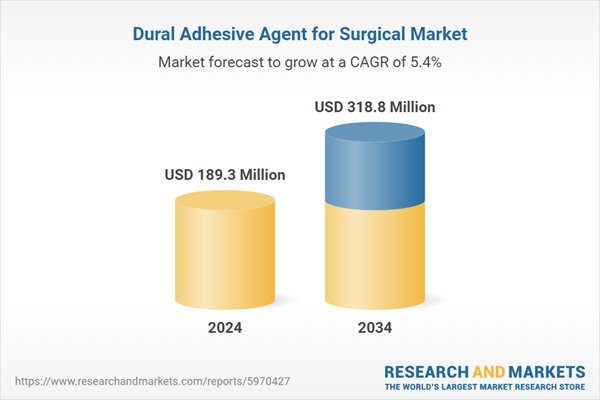
| Estimated Market Value ( USD | $ 189.3 Million |
| Forecasted Market Value ( USD | $ 318.8 Million |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |