Market Drivers of the Hyperpigmentation Disorder Treatment Market
Rise in Prevalence of Melasma
Melasma, one of the most dominant hyperpigmentation conditions, is characterized by symmetrical light to dark muddy-brown macules with borders on the face, particularly the chin, upper lip, nose, forehead, and cheeks. It is prevalent among women and those with darker complexion. Malar, mandibular, and centrofacial clinical features are common in melasma. Sun exposure, hormonal variations, pregnancy, hereditary factors, cosmetics, and medicines have all been linked to the development of melasma. According to a study published in Elsevier BV in September 2023, Various studies have estimated that the overall prevalence of melasma in the general population ranges from 1% to 50%.%. 55-64% of melasma cases have reported a family history of the same condition. Moreover, a greater prevalence is reported in individuals of East Asian and Hispanic origins as well as in patients with high Fitzpatrick skin phototypes (IV to VI); it is also higher in individuals living in locations that receive intense UV radiation.Pregnancy is another common cause of melasma. This condition also impacts women taking oral contraceptives and hormones. Estrogen, progesterone, and melanocyte-stimulating hormone levels normally rise during the third trimester of pregnancy, which may be a factor. According to the National Center for Biotechnology Information (NCBI) in August 2023, melasma is more common in reproductive years, and it rarely occurs before puberty; the condition occurs in 15-50% of pregnant women, and its prevalence ranges from 1.5% to 33%, depending on the population in a different region. Therefore, an upsurge in melasma prevalence triggers the demand for hyperpigmentation treatments.
Opportunities in the Hyperpigmentation disorder treatment Market
Major hyperpigmentation disorder treatment market players focus on research and development activities to develop and launch innovative and efficient products. They are also making efforts to win regulatory approvals for their products. Some of the recent product developments and launches, which are likely to create ample growth opportunities for market players, are mentioned below.- In October 2022, Galderma introduced Skincare A-LUMINATE BRIGHTENING SERUM, a breakthrough product in the skincare industry. This innovative addition to the ALASTIN Skincare range is specifically formulated to lower the appearance of surface hyperpigmentation without the use of harsh or irritating ingredients.
- In February 2022, Alchemee introduced the new Restorative Elements brand to help in the correction of the appearance of various types of skin discoloration and hyperpigmentation symptoms, including age spots, sun spots, uneven skin tone, and post-inflammatory hyperpigmentation (PIH). The Restorative Elements is a dermatologist-developed, clinically-proven routine formulated to safely and gently help fade the visible signs of skin hyperpigmentation.
- In March 2023, Candela Corporation won the US Food and Drug Administration (FDA) approval for its PicoWay laser designed to treat café au lait, lenHtigines, melasma, and Nevus of Ota.
- In May 2022, Scientis launched the Cyspera Intensive System, a three-product system formulated to improve hyperpigmentation.
Hyperpigmentation disorder treatment Market: Segmental Overview
By treatment type, the market is segmented into cosmeceutical, light or laser therapy, microdermabrasion, chemical peels, cryotherapy, and others. The cosmeceutical segment held the largest hyperpigmentation disorder treatment market share in 2022. Further, the microdermabrasion segment is anticipated to register the highest CAGR during the forecast period.The market, by condition, is categorized into melasma, solar lentigines, post-inflammatory hyperpigmentation, and others. The melasma segment held the largest hyperpigmentation disorder treatment market share in 2022. It is further anticipated to register the highest CAGR during the forecast period.
The market, by end user, is categorized into hospitals, dermatology centers, and others. The hospitals segment held the largest market share in 2022, Further, the dermatology centers segment is anticipated to register the highest CAGR during the forecast period.
Hyperpigmentation disorder treatment Market: Geographical Overview
In 2022, North America held the largest share of the market. The increasing numbers of clinics and hospitals providing hyperpigmentation disorder treatment services, and an evolving demographics of the US with the growth of Hispanic population with a dark skin type is driving the growth of the market. Other factors such as the increasing number of product launches and approvals, the surging number of aesthetic clinics, and growing access to numerous hyperpigmentation treatments propel the hyperpigmentation disorder treatment market growth in Canada. For instance, in June 2023, Sun Pharma Canada Inc. received Health Canada's approval of PRABSORICA LD (isotretinoin capsules). ABSORICA LD is a new and only micronized formulation of isotretinoin, which is the first formulation developed in over a decade to treat severe acne in patients aged 12 and older.Table of Contents
Companies Mentioned
- Abbvie Inc.
- Bayer AG
- Epipharm AG
- Galderma Laboratories
- Obagi Cosmeceuticals LLC
- Lutronic Corporation
- La Pristine
- L’oreal S.A
- Pierre Fabre Group
- Vivier Pharma USA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 158 |
| Published | April 2024 |
| Forecast Period | 2022 - 2030 |
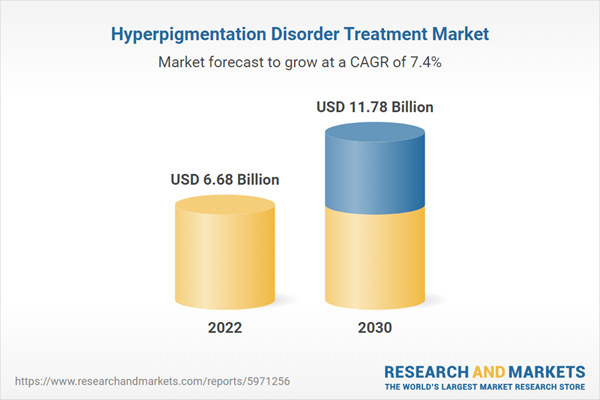
| Estimated Market Value ( USD | $ 6.68 Billion |
| Forecasted Market Value ( USD | $ 11.78 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |