Global Interbody Fusion Cage Market Size
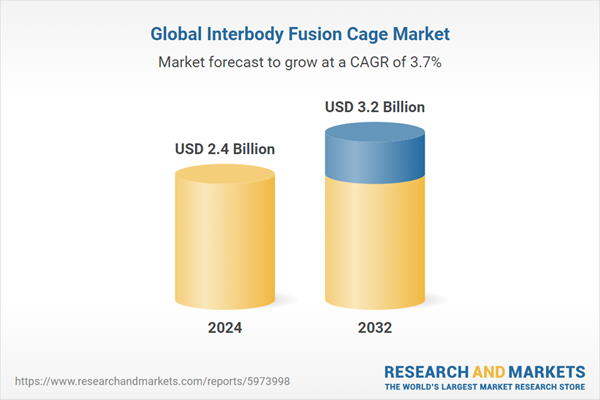
The global interbody fusion cage market was valued at USD 2.3 million in 2023, driven by increasing incidence of spinal cord injuries across the globe. The market is expected to grow at a CAGR of 3.70% during the forecast period of 2024-2032, with the values likely to reach USD 3.2 million by 2032.Global Interbody Fusion Cage Market Outlook
- The demand for interbody fusion cage is on the rise, intended to combat the spinal disorders.
- One of the major market trends includes research and development to produce innovative products that can serve the patients.
- The Asia Pacific region is likely to experience notable growth in the forecast period. Medical tourism in countries like India, China and South Korea play a critical role in boosting the market value.
Global Interbody Fusion Cage Market Overview
Interbody fusion cage is a prosthesis used in spinal fusion procedures to maintain foraminal height and decompression. These cages are small, hollow implants that are placed between the vertebrae where the disc material has been removed. According to World Health Organization (WHO), over 15 million people are living with spinal cord injury (SCI). Individuals with SCI are at a risk of developing debilitating and even life-threatening secondary conditions, which can cause premature mortality.Global Interbody Fusion Cage Market Growth Drivers
Increasing Funding for Research and Development to Affect the Market Landscape Significantly
In February 2024, a project at University of Pittsburgh received fundings worth USD 557,000 from NIH grant to develop "metamaterial" orthopedic implants for spinal treatments. Metamaterial implants for the spine aim to offer enhanced mechanical compatibility and potentially reducing the likelihood of rejection and other complications. The successful development and clinical application of these metamaterial implants could greatly impact spinal treatment, potentially offering more effective and less invasive options.Growth in Strategic Collaborations and Mergers to Meet Rising Interbody Fusion Cage Market Demand
In October 2023, NanoHive Medical and DirectSync Surgical announced a collaboration. It was focused on integrating NanoHive Medical's nanotechnology expertise with DirectSync Surgical's capabilities in spinal surgery devices and create a new generation of spinal implants that are both smart and powered by patient activity. The strategic collaboration is a joint development to create a first-of-kind stimulating/sensing 3D printed interbody fusion cage. The SmartSpinal implants features embedded sensors and energy-harvesting technologies that utilize the patient's own movements to power the device.Global Interbody Fusion Cage Market Trends
The market is witnessing several trends and developments to improve the current global scenario. Some of the notable trends are as follows:Technological Advancements
The market is experiencing a shift towards using biocompatible material that enhances osseointegration, such as titanium. Moreover, the integration of porous or 3D-printed structures are gaining popularity as they mimic bone architecture and encourage natural bone growth. One of the advancements in medical science is 3D printing. This technology allows for customization of cages that can fit the anatomy of an individual patient.Increased Adoption of Minimally Invasive Techniques
Minimally invasive surgeries are gaining traction as it reduces duration of stay at hospitals. It also provides multiple benefits including lower complications, and faster recovery time. The surgeries that are minimally invasive often incorporate advanced surface technologies to enhance fusion rates and stability in the absence of large, open procedures.Rising Prevalence of Spinal Disorders
Globally, the aging population is increasing, due to which the incidence of spinal injuries is rising and hence acts as a driver for the growth of interbody fusion cage market. Due to the susceptibility of the geriatric population to chronic back pain and degenerative spinal conditions, the demand for innovative spinal fusion products is rising along with practice of minimally invasive surgeries.Expanding Indications for Use
Primarily, interbody fusion cage was used in degenerative disc disease, spinal stenosis, or scoliosis. However, its application has broadened over the period of time. With research and technology, it is now used in surgeries to improve spinal stability and alignment. This expansion is expected to boost market growth as it caters to several patient demographics.Global Interbody Fusion Cage Market Segmentation
Market Breakup by Product Type
- Lumbar Cage
- Cervical Cage
- Thoraco-lumbar
- Cage Thoracic Cage
Market Breakup by Surgery
- Anterior
- Posterior
- Lateral
- Transforaminal
Market Breakup by End User
- Hospitals & Clinics
- Ambulatory Surgical Center
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Interbody Fusion Cage Market Share
Market Segmentation Based on Product Types is Anticipated to Witness Substantial Growth
Based on product type, the market segmentation includes lumbar cage, cervical cage, thoraco-lumbar, and cage thoracic cage. The lumbar cage dominates the market as it is primarily used in surgeries involving the lower spine. Its market value is driven by high incidence of lumbar degenerative diseases and increasing geriatric population, which is more susceptible to lower back issues.The cervical spine at the neck region is addressed with a cervical cage. The adoption of minimally invasive surgeries is increasing along with the prevalence of trauma cases, which is boosting the market share of this segment significantly.
Surgery Types are Expected to Boost Interbody Fusion Cage Market Value
Based on surgery, the market constitutes anterior, posterior, lateral, and transforaminal. Each surgery type addresses a different anatomical area and requires different surgical requirements. The anterior approach involves accessing the spine from the front and is used for cervical and lumbar regions. This approach gives an advantage of providing direct access to the spine while minimizing damage to the surrounding muscular and neural structures. These surgeries are effective in treating complex spinal disorders and improve fusion rates and patient outcomes.Posterior surgeries are common due to their applicability in various spinal regions and conditions requiring access through the back of the spine. The preference for this segment can be attributed to familiarity its proven safety and efficacy profiles. Moreover, research initiatives have led to the introduction of novel materials like peek and titanium in cage designs. This enhances success rates of the procedure, and is likely to bolster market growth during the forecast period.
Global Interbody Fusion Cage Market Analysis by Region
Regionally, the market report offers an insight into North America, Europe, Asia Pacific, Latin America, Middle East, and Africa. With high prevalence of spinal disorders, presence of an advanced healthcare infrastructure and rapid adoption of new technologies, North America is expected to lead the global market share.Europe is also projected to hold a significant market value in the forecast period. The presence of geriatric population along with prominent academic institutions boost its market share. In the forecast period, Asia Pacific is projected to witness fastest growth. The developing healthcare infrastructure, increasing awareness of new surgeries, and growing medical tourism is contributing to the expansion of the market. Growth at economical level facilitates better healthcare and thereby market growth.
Leading Players in the Global Interbody Fusion Cage Market
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Zimmer Biomet
- Precision Spine USA
- B. Braun Melsungen AG
- Medacta International
- SpineArt SA
- Stryker
- Ulrich Medical
- NuVasive, Inc
- Medtronic Plc.
- Orthofix US LLC
- Alphatec Spine Inc.
- Aurora Spine, Inc.
- Johnson and Johnson
Key Questions Answered in the Global Interbody Fusion Cage Market Report
- What was the global interbody fusion cage market value in 2023?
- What is the global interbody fusion cage market forecast outlook for 2024-2032?
- What are the regional markets covered in the report?
- What is market segmentation based on product type?
- What is the market breakup based on surgery?
- Who are the major end users in the market?
- What are the major factors aiding the global interbody fusion cage market demand?
- How has the interbody fusion cage market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- How does the prevalence and incidence of spinal disorders and injuries affect the market landscape?
- What are the major global interbody fusion cage market trends?
- How does the rise in the geriatric population impact the market size?
- What product type will dominate the market share?
- Who are the key players involved in the interbody fusion cage market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers and acquisitions among the key market players shaping the market dynamics?
Meta description
The global interbody fusion cage market size was valued at USD 2.3 million in 2023 and it is anticipated to grow at a CAGR of 4.05% during the forecast period of 2024-2032.This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Zimmer Biomet
- Precision Spine USA
- B. Braun Melsungen AG
- Medacta International
- SpineArt SA
- Stryker
- Ulrich Medical
- NuVasive, Inc
- Medtronic Plc.
- Orthofix US LLC
- Alphatec Spine Inc.
- Aurora Spine, Inc.
- Johnson and Johnson
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 2.4 Billion |
| Forecasted Market Value ( USD | $ 3.2 Billion |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |