Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Surgeon Training and Expertise
In Spain, the Minimally Invasive Surgical Devices Market is experiencing robust growth, and one of the key drivers behind this expansion is the growing emphasis on surgeon training and expertise in minimally invasive surgery (MIS). Minimally invasive procedures have gained popularity due to their potential to reduce patient trauma, accelerate recovery, and improve overall outcomes.The cornerstone of minimally invasive surgery is the development of highly specialized skills among surgeons. These skills encompass precise hand-eye coordination, an in-depth understanding of advanced surgical instruments, and the ability to navigate complex anatomical structures with finesse. Surgeons trained in MIS techniques can perform a wide range of procedures with smaller incisions, reducing patient trauma and post-operative pain.
As surgeons become proficient in minimally invasive techniques, they can apply these skills to an increasing number of medical specialties. Originally associated with fields like laparoscopy and endoscopy, MIS is now widely utilized in gynecology, urology, orthopedics, and more. The diversification of minimally invasive procedures opens up new opportunities for surgical device manufacturers, as each specialty requires specific instruments and tools tailored to their unique needs.
Patients have a growing awareness of the advantages of minimally invasive surgery, including quicker recovery times and reduced pain. Surgeons who are well-versed in MIS techniques inspire greater confidence among patients. This trust can drive an increased demand for minimally invasive procedures, pushing healthcare providers to invest in the latest surgical devices to meet patient expectations.
The medical community's commitment to continuous learning and knowledge sharing is a significant driver of MIS expertise. Surgeons often attend workshops, conferences, and training programs to stay up to date with the latest advancements in minimally invasive surgery. This culture of collaboration and knowledge exchange promotes the adoption of new techniques and technologies, creating fertile ground for the growth of the Minimally Invasive Surgical Devices Market.
The training and expertise of surgeons also play a crucial role in driving innovation in surgical devices. Surgeons' feedback and insights are invaluable for the development of new and improved minimally invasive instruments. They can provide practical perspectives on what works best in the operating room, leading to the creation of more effective, efficient, and user-friendly devices.
Patient Preferences
Spain's Minimally Invasive Surgical Devices Market is witnessing remarkable growth, and one of the key factors propelling this expansion is the increasing significance of patient preferences. Patients are becoming more discerning and proactive in their healthcare choices, and their preference for minimally invasive surgery (MIS) is influencing the market's development.Patients today are better informed about healthcare options, and they often prefer minimally invasive surgery because it offers a less invasive and disruptive experience compared to traditional open surgery. Minimally invasive procedures typically involve smaller incisions, resulting in reduced post-operative pain, shorter hospital stays, and quicker recovery times. These benefits align with patients' desires for a more comfortable and convenient healthcare experience.
In many cases, minimally invasive procedures are associated with better cosmetic outcomes. Smaller incisions leave minimal scarring, which is a significant consideration for patients, especially when the surgical site is in a visible location. Patients often favor MIS techniques for their potential to enhance their appearance while addressing medical concerns.
Pain management is a critical aspect of patient care, and minimally invasive surgery is known for reducing post-operative pain and discomfort. Patients who have experienced less pain during recovery are more likely to opt for minimally invasive procedures in the future, boosting the demand for surgical devices that facilitate these techniques.
Patient preferences are heavily influenced by the desire to return to their daily lives as swiftly as possible. Minimally invasive surgery allows for faster recovery and a shorter interruption to a patient's routine. This appeals to those who are eager to resume their work, family responsibilities, and recreational activities.
Healthcare providers are increasingly adopting a patient-centered approach to care, focusing on individual patient needs and preferences. Surgeons who take these preferences into account are more likely to recommend minimally invasive options when appropriate. The alignment of patient and provider priorities contributes to the market's growth.
Patients tend to have more trust and confidence in surgical techniques that align with their preferences. When surgeons recommend minimally invasive options, patients are more likely to comply with the recommended treatment plans, fostering better patient-surgeon relationships. This trust not only drives the growth of MIS procedures but also the demand for advanced surgical devices.
Satisfied patients often share their positive experiences with friends and family, leading to word-of-mouth recommendations. These recommendations can further enhance the popularity of minimally invasive surgery, spurring the adoption of advanced surgical devices.
Healthcare Costs
Spain's Minimally Invasive Surgical Devices Market is experiencing significant growth, driven by a multitude of factors. Among these, a crucial driver is the ability of minimally invasive surgery (MIS) to reduce healthcare costs. As the healthcare industry worldwide seeks more efficient and cost-effective solutions, the adoption of MIS techniques is becoming increasingly attractive.Minimally invasive surgery often leads to shorter hospital stays compared to traditional open surgeries. Patients who undergo minimally invasive procedures typically recover faster and experience fewer complications, allowing them to leave the hospital sooner. This reduced length of stay translates into cost savings for healthcare facilities, as resources can be allocated more efficiently.
Minimally invasive surgery is associated with decreased post-operative care expenses. Patients who experience less pain and a smoother recovery process generally require fewer medications, follow-up appointments, and home care services. This results in a reduction in overall healthcare expenditure.
MIS procedures generally use fewer healthcare resources, including medications, surgical supplies, and personnel. Smaller incisions and less invasive techniques mean fewer resources are required during the surgical procedure and recovery phase. This economizes healthcare resources, making them available for other patients and needs.
Minimally invasive surgery is known to reduce the risk of complications and the need for hospital readmissions. Patients undergoing traditional open surgery may experience complications that necessitate further hospitalization and treatment. MIS techniques help mitigate these risks, resulting in lower healthcare costs associated with complications and re-admissions.
Minimally invasive procedures are often more efficient and require less time in the operating room compared to open surgeries. This reduction in operating room time allows healthcare facilities to schedule more surgeries within the same time frame, thereby optimizing their resources and reducing overhead costs.
The faster recovery associated with MIS procedures allows patients to return to their daily lives and work sooner. This not only benefits patients but also helps maintain a productive workforce. Reduced downtime for patients results in lower indirect costs for employers and society as a whole.
Advancements in Imaging Technology
Spain's Minimally Invasive Surgical Devices Market is experiencing rapid growth, driven by numerous factors, with advancements in imaging technology playing a pivotal role. Minimally invasive surgery (MIS) relies heavily on high-quality imaging to enable surgeons to navigate intricate anatomical structures with precision.One of the primary benefits of advanced imaging technology is the ability to provide surgeons with high-definition, real-time images of the surgical site. This enhanced precision allows surgeons to perform complex procedures with greater accuracy, reducing the risk of complications and improving patient outcomes. The demand for such precision drives the adoption of sophisticated surgical devices designed to work in conjunction with cutting-edge imaging systems.
Modern imaging technology often includes 3D visualization capabilities, offering a more comprehensive view of the surgical field. This 3D perspective allows surgeons to perceive depth and spatial relationships more accurately, making it easier to navigate challenging anatomical structures. As 3D visualization becomes more accessible, the demand for compatible minimally invasive surgical devices increases.
Advanced imaging technology not only aids during surgery but also enhances pre-operative diagnostics. High-quality imaging, such as MRI, CT scans, and ultrasound, can help surgeons better plan their procedures, identify pathology, and create a detailed roadmap for minimally invasive surgeries. This, in turn, fuels the demand for specialized devices that can work seamlessly with advanced imaging systems.
Real-time imaging feedback allows surgeons to adjust their techniques and make informed decisions during the procedure. This capability enhances the surgeon's confidence and the patient's safety. Surgical devices equipped with real-time feedback mechanisms, such as robotic-assisted surgical systems, are in high demand due to their potential to optimize surgical outcomes.
Advancements in imaging technology are expanding the applications of minimally invasive surgery. Procedures that were previously considered too complex to perform minimally invasively are now feasible due to improved imaging capabilities. This expansion of the scope of MIS drives the need for a wider range of specialized devices.
Key Market Challenges
Access and Equity
The benefits of minimally invasive surgery are not evenly distributed across all regions and demographics. Rural or underserved areas may lack access to the necessary equipment and expertise, leading to disparities in healthcare. Ensuring equitable access to MIS procedures and devices remains a significant challenge.Maintenance and Upkeep
Once surgical devices are acquired, ongoing maintenance and upkeep are essential to ensure their proper functioning. Routine maintenance can be costly and time-consuming, and facilities need to allocate resources for these tasks to prevent equipment downtime.Competition
The Minimally Invasive Surgical Devices Market is highly competitive, with numerous companies vying for market share. The competition can lead to price pressures, as well as the need for continuous innovation to stay ahead in the market.Key Market Trends
Robotic-Assisted Surgery
Robotic-assisted surgery has already made significant inroads in Spain, and this trend is expected to continue. The use of surgical robots enhances the precision and dexterity of surgeons, making complex minimally invasive procedures more accessible. These systems offer 3D visualization, real-time feedback, and the ability to perform surgeries remotely. As technology improves and becomes more cost-effective, we can anticipate wider adoption of robotic-assisted surgery in Spain.Miniaturization and Micro-Instruments
The trend towards miniaturization is set to continue, with the development of smaller, more precise surgical instruments and devices. These micro-instruments allow for even less invasive procedures, reducing trauma to the patient and speeding up recovery. Such instruments are particularly relevant in fields like neurosurgery, where precision is paramount.Single-Incision Surgery
Single-incision surgery, also known as scarless surgery, is on the rise. This technique involves performing surgeries through a single small incision, often in the patient's navel, to minimize scarring. As the technology and instruments for single-incision procedures improve, more patients will opt for this less invasive approach.Segmental Insights
Type Insights
Based on Type, Surgical scopes are poised to dominate the Minimally Invasive Surgical Devices Market in Spain. First and foremost, they offer precision and accuracy, allowing surgeons to perform intricate procedures with greater confidence. Additionally, surgical scopes enable reduced tissue trauma and faster patient recovery times, aligning with the growing demand for minimally invasive surgical techniques in Spain's healthcare landscape. Also, technological advancements have enhanced the capabilities of surgical scopes, such as high-definition imaging and advanced ergonomics, ensuring superior visualization and user comfort. The increasing prevalence of chronic diseases and the aging population in Spain are also driving the demand for minimally invasive surgical procedures, creating a favorable market environment for surgical scopes. With these factors in play, surgical scopes are well-positioned to maintain a dominant presence in the Spanish Minimally Invasive Surgical Devices Market.Regional Insights
The Central Region of North Spain is poised to dominate the Minimally Invasive Surgical Devices Market in the country. First, this region serves as a healthcare hub, housing numerous renowned medical facilities and research institutions, which are at the forefront of adopting cutting-edge medical technologies and techniques. These institutions draw highly skilled medical professionals and researchers, making them early adopters of minimally invasive surgical devices. Additionally, the Central Region's strategic geographic location makes it accessible to a wide catchment area, attracting patients from various parts of the country and even from neighboring regions. Likewise, the region's robust infrastructure and transportation networks ensure efficient distribution and access to medical supplies, further boosting its prominence in the market. With a combination of advanced medical expertise, accessibility, and infrastructure, the Central Region of North Spain is well-positioned to lead in the adoption and utilization of minimally invasive surgical devices within the Spanish healthcare landscape.Key Market Players
- Medtronic plc
- Stryker Iberia S.L.
- B. Braun Medical, S.A.
- Olympus Iberia S.A.U
- Boston Scientific España
- Johnson & Johnson S.A.
- Karl Storz Endoscopy Iberica S A
- Abbott Laboratories S.A.
- Koninklijke Philips N.V.
- Conmed Corporation
Report Scope:
In this report, the Spain Minimally Invasive Surgical Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Spain Minimally Invasive Surgical Devices Market, By Type:
- Handheld Instruments
- Graspers
- Retractors/Elevators
- Dilators
- Suturing Instruments
- Others
- Surgical Scopes
- Laparoscopes
- Gastroscope
- Cystoscope
- Ureteroscope
- Others
- Cutting Instruments
- Trocar’s
- Other MIS instruments
- Guiding Devices
- Guiding Catheters
- Guidewires
- Electrosurgical Devices
- Electrosurgery Instruments & Accessories
- Electrosurgery Generators
- Patient Return Electrodes
- Others
Spain Minimally Invasive Surgical Devices Market, By Surgery Type:
- Cardiovascular
- Gastrointestinal
- Gynecology
- Urology
- Others
Spain Minimally Invasive Surgical Devices Market, By End User:
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Others
Spain Minimally Invasive Surgical Devices Market, By Region:
- Central Region North Spain
- Aragon & Catalonia
- Andalusia, Murcia & Valencia
- Madrid, Extremadura & Castilla
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Spain Minimally Invasive Surgical Devices Market.Available Customizations:
Spain Minimally Invasive Surgical Devices market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Medtronic plc
- Stryker Iberia S.L.
- B. Braun Medical, S.A.
- Olympus Iberia S.A.U
- Boston Scientific España
- Johnson & Johnson S.A.
- Karl Storz Endoscopy Iberica S A
- Abbott Laboratories S.A.
- Koninklijke Philips N.V.
- Conmed Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 87 |
| Published | June 2024 |
| Forecast Period | 2024 - 2029 |
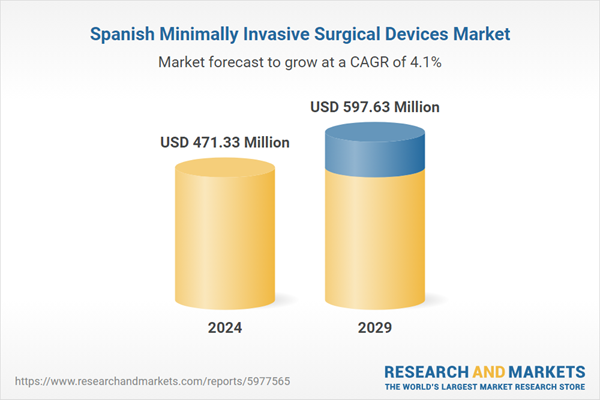
| Estimated Market Value ( USD | $ 471.33 Million |
| Forecasted Market Value ( USD | $ 597.63 Million |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Spain |
| No. of Companies Mentioned | 10 |