In neurological testing, brain monitoring systems are used to identify any irregularity in the functionality of brain cells (neurons) that contributes to the development of neurological illnesses such as Parkinson's disease, epilepsy, Alzheimer's, and sleep problems. These technologies enable the monitoring and detection of neuronal activity without the need for invasive neurosurgery. Monitoring patients with traumatic brain injuries is becoming increasingly important to avoid further brain damage, especially cerebral ischemia.
The rising need for evaluating elevated intracranial pressure (ICP) in monitoring patients will drive demand for brain monitoring devices. According to the US National Institute of Mental Health (NIMH) 2020, one in every four American individuals has a diagnosable mental condition in any given year, with almost 6% having a severe disability. Additionally, according to the same estimate, the overall annual cost of serious mental illness in the United States surpasses USD 317 billion. Therefore, this would also lead the market to grow during the projected period.
The brain monitoring market is relatively competitive, with both large and small industry participants. In terms of market share, a small number of significant firms now dominate the market analyzed in certain areas. However, with technological improvements and product breakthroughs, mid-size to smaller businesses have increased their market presence by bringing new, more usable technologies.
Market Drivers:
Aging demographics, increased prevalence of brain illnesses, and gadget simplicity would propel the market growth.
In the United States, traumatic brain injury (TBI) is a leading cause of mortality and disability. TBIs account for around 30% of all injury mortality. Every day, 153 people in the United States die from injuries that include TBI (source: Centers for Disease Control and Prevention). In addition, rising healthcare expenditure and high incidences of neurological disorders due to changing living conditions and increasing stress levels are driving the market growth.Furthermore, the growing technological advancements will provide an opportunity for the development of brain sensors, which will boost the growth of global brain monitoring devices in the forecast period. Moreover, the first versions of EEG monitors were filled with cables and electrodes, and even today, the great majority of devices are based on this traditional architecture. However, with the introduction of wireless connectivity, the size and awkwardness of EEG monitors and other associated equipment have been greatly decreased.
Rising Innovation in the devices is anticipated to increase the demand for brain monitoring systems.
The rising prevalence of various neurodegenerative and neurological disorders has led to an increased frequency of innovation in mobile EEG devices, real-time monitoring, and alarms, which is thus contributing to the demand for brain monitoring products. According to the National Institute of Neurological Disorders and Stroke, about 30 million people in the US alone suffer from at least one of the nearly 7,000 identified uncommon or ultra-rare disorders. More than 45% of uncommon diseases are associated with neurological symptoms. Factors like this are thus expected to surge the demand for brain monitoring products thus driving the market.Furthermore, a rising number of regulatory approvals for brain health devices is projected to drive the global brain monitoring systems market growth. Several hospitals and healthcare organizations implement the insertion of brain implants in patients who are at risk of developing neurological disorders to provide artificial control on brain functioning. Additionally, regulatory approvals for innovative and advanced brain health devices are predicted to propel the market growth in the near future. For instance, in April 2019, GE Healthcare received the US Food and Drug Administration 510(k) clearance for its Deep Learning Image Reconstruction engine on the new Revolution Apex computed tomography device.
Market Key Developments:
- January 2024-The National Stem Cell Foundation (NSCF) is using the International Space Station's microgravity environment to study brain organoids from patients with Parkinson's and PPMS. The mission, marking NSCF's fifth flight, is to collect data on tissue changes and inflammation in the brain. The data will inform the foundation's next mission, which will include organoids from induced pluripotent stem cells from affected patients. A follow-on investigation will be launched on SpaceX's 30th Commercial Resupply Services mission, including organoids from patients with Alzheimer's disease.
- November 2022-The FDA approved the Neurosteer single-channel EEG brain monitoring platform for clinical use. This platform allows for continuous monitoring of presymptomatic cognitive decline in diseases like Alzheimer's, Parkinson's, and dementia. The platform can also aid in clinical trials for pharmaceutical therapeutics, facilitating cost-effective mass screening of neurodegenerative patients. The short set-up time and machine-learning features make it objective and easy to assess cognitive states. The portable and affordable EEG system is now widely available to all populations.
- October 2022-Medtronic launched the Medtronic Neurovascular Co-Lab™, an innovation process and platform for new ideas in stroke care. The platform collects and examines ideas from doctors, inventors, entrepreneurs, and startups to improve stroke care. A team analyzes the ideas and expedites the best ones for investment in research or prototype development. The platform can also help shepherd therapies through clinical and commercial stages. It highlighted two big needs for innovation in stroke care: innovations to extend the window of care and discover ways to foster brain tissue regrowth.
- February 2022-Masimo received FDA clearance for SedLine® brain function monitoring for pediatric patients aged 1-17 years and the SedLine Pediatric EEG Sensor. This expansion of SedLine's potential benefits to all patients one year old and above in the United States is a significant step towards improving the quality of care for patients with challenging and youngest brains. SedLine uses advanced signal processing technology to monitor brain activity bilaterally, providing clinicians with a more complete picture of the brain, helping to personalize sedoanalgesia and detect warning signs.
Company Products:
- Bispectral Index™ (BIS™)is a monitoring technique that enhances the clinician’s patient-targeted approach to induction, maintenance, and emergence. BIS™ technology non-invasively measures and interprets the wave activity of the brain directly related to the effects of anesthetic agents.
- SedLine®Brain Function Monitoringis a brain monitoring system that helps clinicians monitor the state of the brain under anesthesia. It acquires bilateral data and processes EEG signals that help clinicians with anesthetic management.
Segmentation:
By Product Type
- Electroencephalography (EEG)
- Magnetoencephalography (MEG)
- Magnetic Resonance Imaging (MRI)
- Cerebral Oximeters
- Others
By Application
- Traumatic Brain Injury (TBI)
- Epilepsy
- Alzheimer’s Disease
- Others
By End-user
- Hospitals & Clinics
- Neurological Centers
- Academic and Research Institutes
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- Japan
- South Korea
- India
- Indonesia
- Thailand
- Others
Table of Contents
Companies Mentioned
- Compumedics Limited
- GENERAL ELECTRIC COMPANY
- Medtronic
- Natus Medical Incorporated
- NIHON KOHDEN CORPORATION
- Tristan Technologies, Inc.
- Masimo Corporation
- Canon Medical Systems Corporation
- CortiCare, Inc.
- NeuroWave Systems Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 138 |
| Published | April 2024 |
| Forecast Period | 2022 - 2029 |
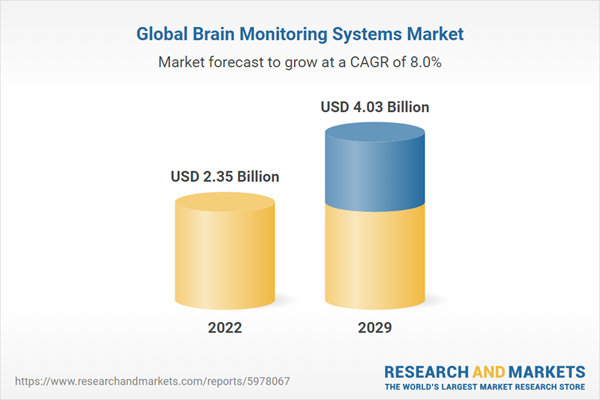
| Estimated Market Value ( USD | $ 2.35 Billion |
| Forecasted Market Value ( USD | $ 4.03 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |