The Germany market dominated the Europe Laboratory Developed Tests Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of$1.46 billion by 2031. The UK market is exhibiting a CAGR of 6.5% during (2024 - 2031). Additionally, The France market would experience a CAGR of 7.3% during (2024 - 2031).
LDTs are used to identify and characterize several infectious diseases, such as fungi, viruses, bacteria, and parasites. These tests are essential for diagnosing infectious disorders, tracking outbreaks, observing patterns in antibiotic resistance, and the direction of infection control protocols in hospital environments.
Various factors, including clinical need, technological advancements, regulatory frameworks, and market dynamics, have driven the adoption of LDTs. Healthcare providers, laboratories, and patients have increasingly recognized the value of LDTs in enhancing diagnostic accuracy, guiding treatment decisions, and improving patient outcomes. LDTs offer unique customization, flexibility, and innovation advantages compared to commercially available diagnostic tests.
The prevalence of genetic disorders in Europe drives a greater demand for genetic testing services. As per the Eurodis, an estimated 30 million people live with a rare disease in 48 European countries. 70% of patients with rare conditions seek medical attention and then wait more than a year for a definitive diagnosis. Also, the growing investment in rare diseases in Europe leads to an increased demand for specialized diagnostic testing tailored to rare disorders’ unique genetic, molecular, and clinical characteristics. As per the European Commission, the EU has extensively supported research in rare diseases through its research and innovation framework programs. Under the 7th Framework Programme and Horizon 2020, about €2.4 billion in funding has been awarded to over 440 worldwide research projects on rare disorders. Therefore, the high prevalence of rare diseases and increasing investment in research projects drive the market’s growth.
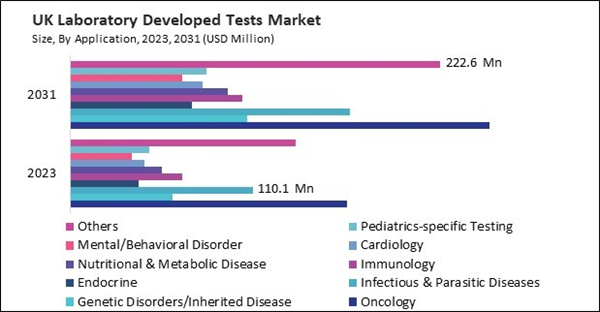
Based on Application, the market is segmented into Oncology, Genetic Disorders/Inherited Disease, Infectious & Parasitic Diseases, Endocrine, Immunology, Nutritional & Metabolic Disease, Cardiology, Mental/Behavioral Disorder, Pediatrics-specific Testing, and Others. Based on Technology, the market is segmented into Molecular Diagnostics, Immunoassays, Hematology & Coagulation, Microbiology, Clinical Chemistry, Histology/Cytology, Flow Cytometry, Mass Spectroscopy, and Others. Based on countries, the market is segmented into Germany, UK, France, Russia, Spain, Italy, and Rest of Europe.
List of Key Companies Profiled
- Neogenomics, Inc.
- Guardant Health, Inc.
- Qiagen N.V
- Quest Diagnostics Incorporated
- Abbott Laboratories
- Siemens Healthineers AG (Siemens AG)
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- F.Hoffmann-La Roche Ltd.
- Eurofins Scientific SE
Market Report Segmentation
By Application- Oncology
- Genetic Disorders/Inherited Disease
- Infectious & Parasitic Diseases
- Endocrine
- Immunology
- Nutritional & Metabolic Disease
- Cardiology
- Mental/Behavioral Disorder
- Pediatrics-specific Testing
- Others
- Molecular Diagnostics
- Immunoassays
- Hematology & Coagulation
- Microbiology
- Clinical Chemistry
- Histology/Cytology
- Flow Cytometry
- Mass Spectroscopy
- Others
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
Table of Contents
Companies Mentioned
- Neogenomics, Inc.
- Guardant Health, Inc.
- Qiagen N.V
- Quest Diagnostics Incorporated
- Abbott Laboratories
- Siemens Healthineers AG (Siemens AG)
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd.
- Eurofins Scientific SE