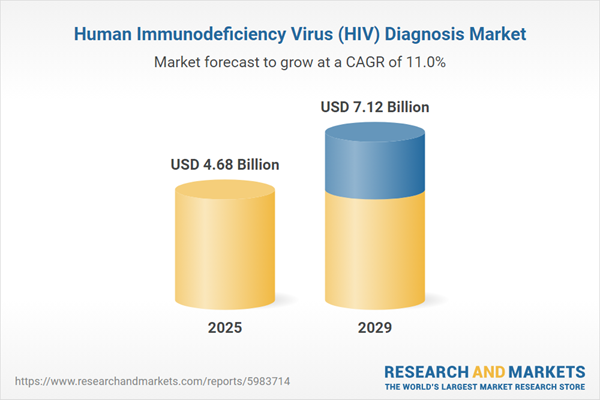
The human immunodeficiency virus (HIV) diagnosis market size has grown rapidly in recent years. It will grow from $4.2 billion in 2024 to $4.68 billion in 2025 at a compound annual growth rate (CAGR) of 11.4%. The growth in the historic period can be attributed to expansion of voluntary counseling and testing, development of HIV antibody tests, emergence of the HIV/AIDS epidemic, and community-led and peer-led initiatives.
The human immunodeficiency virus (HIV) diagnosis market size is expected to see rapid growth in the next few years. It will grow to $7.12 billion in 2029 at a compound annual growth rate (CAGR) of 11%. The growth in the forecast period can be attributed to public health campaigns and awareness initiatives, focus on equity and inclusivity, routine testing in healthcare settings, community-led and peer-led approaches, and expansion of self-testing. Major trends in the forecast period include advancements in testing technologies, focus on targeted testing strategies, community-based testing and outreach, emphasis on equity and inclusivity, and policy and funding priorities.
The anticipated increase in blood donation is poised to drive the expansion of the HIV diagnosis market in the foreseeable future. Blood donation, characterized by the voluntary act of giving blood through phlebotomy, has seen a rise attributed to advancements in medical technology, heightened community engagement, and enhanced donor recruitment and retention strategies. This surge in blood donation results in a larger pool of samples available for HIV testing. Consequently, the heightened availability of blood samples facilitates more comprehensive screening for HIV antibodies or antigens, thereby improving the accuracy and reliability of HIV diagnosis. For example, NHS Blood and Transplant, a UK-based government agency, reported an increase in new blood donors from 119,016 in 2022-2023 to 119,371 in 2023-2024, underscoring the pivotal role of rising blood donation in propelling the growth of the HIV diagnosis market.
Major players in the HIV diagnosis market are actively developing chip-based real-time PCR test solutions tailored for HIV, aimed at enhancing accuracy, reducing turnaround time, and improving accessibility. Chip-based real-time PCR test solutions utilize microfluidic chips or small integrated circuits to conduct real-time polymerase chain reaction (PCR) tests for HIV detection. For instance, in August 2022, Molbio Diagnostics, an India-based molecular diagnostics manufacturer, unveiled the Truenat HIV-1 test, a chip-based real-time PCR test designed for the quantitative detection and diagnosis of human immunodeficiency virus type 1 (HIV-1) in whole blood and plasma. Leveraging Taqman chemistry, this test boasts high primer sensitivity and specificity, requiring minimal sample volume and featuring an intelligent chip with lot-specific data for result quantification, along with contamination- and evaporation-resistant design elements.
In May 2022, Roche, a Switzerland-based biopharmaceutical and diagnostic company, forged a partnership with the Global Fund, aiming to bolster diagnostic infrastructure in low- and middle-income countries. This collaboration seeks to facilitate improved diagnosis and treatment of HIV and tuberculosis within the framework of Roche's Global Access Program and the Global Fund's broader initiatives targeting HIV, tuberculosis, and malaria globally. The Global Fund, an international financing and partnership organization headquartered in Switzerland, focuses on providing grants to strengthen health systems addressing HIV/AIDS.
Major companies operating in the human immunodeficiency virus (HIV) diagnosis market are Thermo Fisher Scientific Inc., Abbott Laboratories, Siemens Healthineers, Roche Diagnostics, Becton Dickinson and Company, Hologic Inc., bioMérieux SA, Beckman Coulter Inc., Bio-Rad Laboratories Inc., QIAGEN N.V., Bio-Techne Corporation, Alere Inc., Cepheid Inc., OraSure Technologies Inc., Advanced Instruments Inc., BBI Solutions, Chembio Diagnostic Systems Inc., BioLytical Laboratories Inc., Avioq Inc., Advanced Biochemicals Ltd., Advanced Molecular Diagnostics, Atila BioSystems Inc., BioAssay Works LLC, Chemtron Biotech Inc.
North America was the largest region in the HIV diagnosis market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the human immunodeficiency virus (HIV) diagnosis market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the human immunodeficiency virus (HIV) diagnosis market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Human immunodeficiency virus (HIV) diagnosis encompasses the process of determining whether an individual has contracted the human immunodeficiency virus, which leads to acquired immunodeficiency syndrome (AIDS). This diagnostic procedure involves testing for the presence of HIV antibodies, antigens, or viral genetic material in bodily fluids such as blood, saliva, or other relevant specimens.
The primary products utilized in HIV diagnosis include consumables, instruments, and software and services. Consumables refer to materials or supplies that are used up during diagnostic procedures, and they can be deployed in various formats including self-tests and lab-based assays. These products find application across diverse end users such as diagnostic laboratories, hospitals, and research institutions.
The HIV diagnosis market research report is one of a series of new reports that provides HIV diagnosis market statistics, including HIV diagnosis industry global market size, regional shares, competitors with a HIV diagnosis market share, detailed HIV diagnosis market segments, market trends and opportunities, and any further data you may need to thrive in the HIV diagnosis industry. This HIV diagnosis market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The human immunodeficiency virus (HIV) diagnosis market consists of revenues earned by entities by providing services such as HIV testing, pre-test counseling, post-test counseling, laboratory-based testing, point-of-care testing, partner notification services, and linkage to care services. The market value includes the value of related goods sold by the service provider or included within the service offering. The human immunodeficiency virus (HIV) diagnosis market also includes sales of diagnostic kits, testing equipment such as enzyme-linked immunosorbent assay (ELISA) readers and polymerase chain reaction (PCR) machines, point-of-care testing devices, laboratory equipment such as centrifuges and microscopes. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Human Immunodeficiency Virus (HIV) Diagnosis Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on human immunodeficiency virus (hiv) diagnosis market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for human immunodeficiency virus (hiv) diagnosis ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The human immunodeficiency virus (hiv) diagnosis market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Consumables; Instruments; Software and Services2) By Mode: Self-Test; Lab-Based
3) By End User: Diagnostic Laboratories; Hospitals; Research Institutions
Subsegments:
1) By Consumables: Test Kits (Rapid Hiv Test Kits, Elisa Test Kits); Reagents and Chemicals; Sample Collection Devices (Blood Collection Tubes, Lancets); Test Strips; Control and Calibration Kits; Disposable Gloves and Safety Equipment2) By Instruments: Hiv Diagnostic Analyzers ( Automated Immunoassay Analyzers); Hiv Viral Load Testing Equipment; Cd4 Count Analyzers; Point-of-Care Hiv Diagnostic Devices; Molecular Diagnostic Instruments ( Pcr Machines); Electrochemical and Optical Hiv Detection Instruments
3) By Software: Hiv Diagnosis Data Management Software; Electronic Health Record (Ehr) Integration Software; Hiv Test Result Interpretation Software; Laboratory Information Management Systems (Lims); Patient Monitoring and Tracking Software; Cloud-Based Hiv Diagnostic Reporting Tools
4) By Services: Diagnostic Testing Services; Hiv Testing Support Services ( Counseling, Education); Diagnostic Lab Services; Telemedicine and Remote Hiv Testing Services; Clinical Trial Services for Hiv Diagnostic Tests; Data Analytics and Interpretation Services for Hiv Testing
Key Companies Mentioned: Thermo Fisher Scientific Inc.; Abbott Laboratories; Siemens Healthineers; Roche Diagnostics; Becton Dickinson and Company
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Human Immunodeficiency Virus (HIV) Diagnosis market report include:- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Siemens Healthineers
- Roche Diagnostics
- Becton Dickinson and Company
- Hologic Inc.
- bioMérieux SA
- Beckman Coulter Inc.
- Bio-Rad Laboratories Inc.
- QIAGEN N.V.
- Bio-Techne Corporation
- Alere Inc.
- Cepheid Inc.
- OraSure Technologies Inc.
- Advanced Instruments Inc.
- BBI Solutions
- Chembio Diagnostic Systems Inc.
- BioLytical Laboratories Inc.
- Avioq Inc.
- Advanced Biochemicals Ltd.
- Advanced Molecular Diagnostics
- Atila BioSystems Inc.
- BioAssay Works LLC
- Chemtron Biotech Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.68 Billion |
| Forecasted Market Value ( USD | $ 7.12 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |