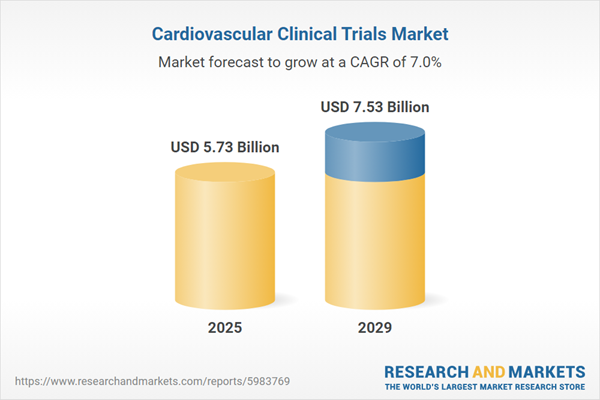
The cardiovascular clinical trials market size has grown strongly in recent years. It will grow from $5.34 billion in 2024 to $5.73 billion in 2025 at a compound annual growth rate (CAGR) of 7.4%. The growth in the historic period can be attributed to increased myocardial infarction, regulatory environment, increased disease burden, advancements in genomics, and increased demand for new drug development.
The cardiovascular clinical trials market size is expected to see strong growth in the next few years. It will grow to $7.53 billion in 2029 at a compound annual growth rate (CAGR) of 7%. The growth in the forecast period can be attributed to an aging population, emerging markets, precision medicine, digital health technologies, and a focus on patient-centric trials. Major trends in the forecast period include increased collaborations, technological advancements, product innovations, new product developments, and the launch of generic versions of combination drugs.
The growth of manufacturing activities is expected to drive the expansion of the extended cord reels market in the coming years. Manufacturing activities involve the processes of producing goods from raw materials or components through various stages of transformation, assembly, and finishing. This increase in manufacturing activities is driven by the adoption of automation and robotics, global market expansion, economic growth in both developed and developing countries, and ongoing investments in research and development. Extended cord reels play a vital role in supporting these activities by providing efficient, safe, and reliable power distribution solutions, which help enhance productivity, safety, and operational efficiency in manufacturing environments. For example, in February 2023, Statistics Canada reported that the manufacturing industry in Canada saw substantial growth, with total sales rising by 17.9% to reach $850.9 billion in 2022. As a result, the rise in manufacturing activities is fueling the demand for extended cord reels.
Leading companies operating in the cardiovascular clinical trials arena are concentrating on forging strategic alliances, such as partnerships with contract research organizations (CROs), to broaden their reach and serve a wider clientele. Collaborating with CROs holds paramount importance in the clinical research sector, facilitating specialized services and support across various stages of clinical trials and research endeavors. For instance, Cereno Scientific AB, a biopharmaceutical firm based in Sweden, recently entered into a partnership with Clinical Trial Consultants (CTC), a full-service CRO in Sweden. This collaboration aims to conduct a Phase I study for CS014, a histone deacetylase inhibitor targeting arterial and venous thrombosis prevention. CTC will play a pivotal role in Phase I trial preparations, including protocol development and clinical trial application processes in Sweden. Scheduled to commence in the first half of 2024, this Phase I trial marks a significant advancement in cardiovascular research efforts.
In February 2023, AstraZeneca, a pharmaceutical company headquartered in the United States, completed the acquisition of CinCor Pharma for $1.8 billion. This strategic acquisition bolsters AstraZeneca's cardiorenal pipeline by incorporating baxdrostat (CIN-107), an aldosterone synthase inhibitor (ASI) designed for blood pressure reduction in individuals with treatment-resistant hypertension. CinCor Pharma, a clinical-stage biopharmaceutical company based in the US, specializes in cardiovascular clinical trials and contributes valuable assets to AstraZeneca's portfolio.
Major companies operating in the cardiovascular clinical trials market are Pfizer Inc., Johnson & Johnson, F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc., AstraZeneca PLC, Novartis AG, Eli Lilly and Company, Gilead Sciences Inc., Amgen Inc., Boehringer Ingelheim International GmbH, Merck & Co. Inc., Baxter International Inc., IQVIA Holdings Inc., SGS S.A., PPD Inc., WuXi AppTec Co. Ltd., Caidya, Syneos Health Inc., Charles River Laboratories International Inc., Sanofi, ICON plc, Medpace Holdings Inc., Cardiovascular Clinical Sciences., ProRelix Services LLP, Worldwide Clinical Trials.
North America was the largest region in the cardiovascular clinical trials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the cardiovascular clinical trials market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the cardiovascular clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Cardiovascular clinical trials are investigations aimed at discovering new methods to prevent, diagnose, treat, and manage cardiovascular illnesses (CVDs) such as coronary artery disease, heart failure, arrhythmias, and hypertension. These studies play a crucial role in advancing medical knowledge and enhancing patient care in the field of cardiology. Clinical trials in this domain typically focus on testing new medications, procedures, or devices related to heart health and stroke outcomes.
The main phases of cardiovascular clinical trials include phase I, phase II, phase III, and phase IV. Phase I clinical trials represent the initial stage of testing a new treatment on a small group of people to evaluate its safety and dosage. These trials may encompass various study designs such as interventional, observational, and expanded access, with indications spanning acute coronary syndrome, coronary artery disease, ischemic heart disease, pulmonary arterial hypertension, stroke, cardiac arrhythmias, heart failure, and others.
The cardiovascular clinical trials market research report is one of a series of new reports that provides cardiovascular clinical trials market statistics, including the cardiovascular clinical trials industry global market size, regional shares, competitors with cardiovascular clinical trials market share, detailed cardiovascular clinical trials market segments, market trends, and opportunities, and any further data you may need to thrive in the cardiovascular clinical trials industry. These cardiovascular clinical trials market research reports deliver a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The cardiovascular clinical trials market includes revenues earned by entities by providing services, such as protocol development, regulatory affairs, and patient recruitment and retention. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Cardiovascular Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on cardiovascular clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for cardiovascular clinical trials ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The cardiovascular clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Phase: Phase I; Phase II; Phase III; Phase IV2) By Study Design: Interventional; Observational; Expanded Access
3) By Indication: Acute Coronary Syndrome; Coronary Artery Disease; Ischemic Heart Disease; Pulmonary Arterial Hypertension; Stroke; Cardiac Arrhythmias; Heart Failure; Other Indications
Subsegments:
1) By Phase I: First-in-Human Trials; Dose Escalation Studies; Safety and Tolerability Studies; Pharmacokinetics and Pharmacodynamics Studies2) By Phase II: Efficacy Studies; Optimal Dosage and Administration Route Studies; Early Safety and Efficacy Trials; Biomarker Development Trials
3) By Phase III: Large-Scale Efficacy Trials; Randomized Controlled Trials (RCTs); Long-Term Safety and Efficacy Studies; Multicenter Trials
4) By Phase IV: Post-Marketing Surveillance; Long-Term Safety Studies; Real-World Evidence (RWE) Studies; Comparative Effectiveness Research
Key Companies Mentioned: Pfizer Inc.; Johnson & Johnson; F. Hoffmann-La Roche Ltd; Thermo Fisher Scientific Inc.; AstraZeneca PLC
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Cardiovascular Clinical Trials market report include:- Pfizer Inc.
- Johnson & Johnson
- F. Hoffmann-La Roche Ltd
- Thermo Fisher Scientific Inc.
- AstraZeneca PLC
- Novartis AG
- Eli Lilly and Company
- Gilead Sciences Inc.
- Amgen Inc.
- Boehringer Ingelheim International GmbH
- Merck & Co. Inc.
- Baxter International Inc.
- IQVIA Holdings Inc.
- SGS S.A.
- PPD Inc.
- WuXi AppTec Co. Ltd.
- Caidya
- Syneos Health Inc.
- Charles River Laboratories International Inc.
- Sanofi
- ICON plc
- Medpace Holdings Inc.
- Cardiovascular Clinical Sciences.
- ProRelix Services LLP
- Worldwide Clinical Trials
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 5.73 Billion |
| Forecasted Market Value ( USD | $ 7.53 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |