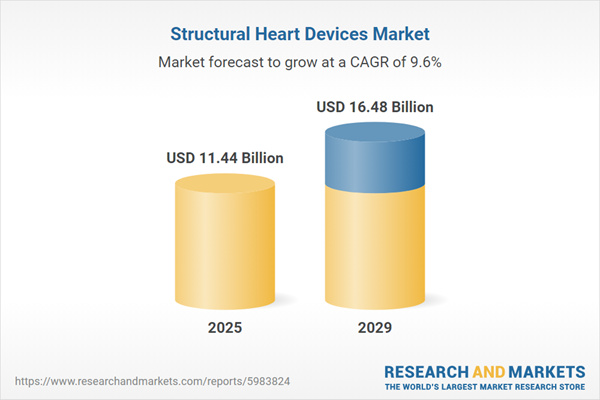
The structural heart devices market size has grown strongly in recent years. It will grow from $10.41 billion in 2024 to $11.44 billion in 2025 at a compound annual growth rate (CAGR) of 9.9%. The growth in the historic period can be attributed to the shift towards less invasive surgical techniques, the demand for minimally invasive structural heart devices, ongoing training and education programs for healthcare professionals, rising healthcare expenditure, growing patient preference for non-surgical or minimally invasive treatment options.
The structural heart devices market size is expected to see strong growth in the next few years. It will grow to $16.48 billion in 2029 at a compound annual growth rate (CAGR) of 9.6%. The growth in the forecast period can be attributed to the increasing aging population, growing awareness about heart health, the implementation of screening programs, favorable reimbursement policies for structural heart procedures, timely regulatory approvals for new structural heart devices. Major trends in the forecast period include increasing adoption of transcatheter valve replacement, ongoing development of innovative devices with advanced materials, growing emphasis on personalized treatment approaches, increasing adoption of hybrid procedures, integration of remote monitoring and telehealth solutions.
The increasing prevalence of cardiovascular diseases is expected to drive the growth of the structural heart device market in the coming years. Cardiovascular diseases (CVDs) encompass a range of conditions that affect the heart and blood vessels, such as coronary artery disease, heart attacks, and strokes. The global incidence of cardiovascular diseases, including heart disease and stroke, has been steadily rising, driven by factors such as sedentary lifestyles, poor diet, obesity, smoking, and aging populations. Structural heart devices are used to treat these diseases by repairing or replacing damaged heart valves, correcting structural defects, and enhancing heart function and patient outcomes. For example, in September 2024, the British Heart Foundation, a UK-based cardiovascular research charity, reported that approximately 7.6 million people in the UK are affected by heart and circulatory diseases, with 4 million males and 3.6 million females living with these conditions. These diseases account for about 27% of all deaths in the UK, resulting in over 170,000 deaths annually, or roughly 480 deaths per day, which equates to one death every three minutes. As a result, the growing prevalence of cardiovascular diseases is fueling the expansion of the structural heart device market.
Leading companies in the structural heart devices market are focusing on developing minimally invasive treatment techniques and devices to improve patient outcomes, shorten recovery periods, and broaden treatment options for structural heart conditions. A notable example is the self-expanding transcatheter aortic valve implantation (TAVI) system, designed to deploy and secure the replacement valve within the native aortic valve anatomy without the need for balloon inflation. For instance, in December 2022, Abbott Laboratories introduced Navitor, the latest-generation transcatheter aortic valve implantation (TAVI) system, in India. Navitor is specifically tailored for individuals with severe aortic stenosis at high or extreme surgical risk and incorporates advancements such as a unique design to prevent valve leakage. This innovation expands Abbott's structural heart transcatheter portfolio, offering both physicians and patients minimally invasive treatment alternatives for heart diseases. Navitor stands out as the sole self-expanding TAVI system with intra-annular leaflets and large frame cells, facilitating improved access to coronary arteries for potential future interventions in coronary artery disease (CAD). Additionally, this new design enhances hemodynamics, optimizing blood flow.
In February 2022, Genesis MedTech Group completed the acquisition of JC Medical (JCM) for an undisclosed sum. This strategic move allows Genesis to augment its product portfolio by including J-Valve, a minimally invasive transcatheter aortic valve replacement (TAVR) device designed for both aortic regurgitation and stenosis patients. JC Medical (JCM) is a US-based company specializing in the manufacture of structural heart devices.
Major companies operating in the structural heart devices market are Abbott Laboratories, Medtronic Plc, Boston Scientific Corporation, Terumo Corporation, Edwards Lifesciences Corporation, W. L. Gore & Associates Inc., Bracco Group, LivaNova PLC, Meril Life Sciences Pvt. Ltd., CryoLife Inc., Braile Biomédica, TTK Healthcare Limited, Direct Flow Medical Inc., JenaValve Technology Inc., Micro Interventional Devices Inc., CardioKinetix Inc., Xeltis AG, Ancora Heart Inc., JOMDD Inc., Valcare Medical, BioStable Science & Engineering Inc., Comed B.V., Transcatheter Technologies GmbH, Navilyst Medical Inc.
North America was the largest region in the structural heart devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the structural heart devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the structural heart devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Structural heart devices are specialized medical implants or tools tailored for treating various structural defects or abnormalities affecting the heart. They are utilized to repair or replace damaged heart structures, such as valves or septa, thereby restoring normal heart functions and enhancing patient outcomes.
The primary types of structural heart devices include heart valve devices, annuloplasty rings, occluders, and delivery systems. Heart valve devices are medical tools crafted to repair or replace damaged heart valves, ensuring proper blood flow through the heart. These procedures encompass replacement procedures, repair procedures for indications such as atrial septal defect, patent foramen ovale, ventricular septal defect, aortic valve stenosis, and others, catering to age group categories including pediatrics and adults.
The structural heart devices market research report is one of a series of new reports that provides structural heart devices market statistics, including structural heart devices industry global market size, regional shares, competitors with a structural heart devices market share, detailed structural heart devices market segments, market trends and opportunities, and any further data you may need to thrive in the structural heart devices industry. This structural heart devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The structural heart devices market consists of sales of paravalvular leak closure devices, endovascular stents, and percutaneous mitral valve replacement. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Structural Heart Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on structural heart devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for structural heart devices ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The structural heart devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Heart Valve Devices; Annuloplasty Rings; Occluders; Delivery Systems2) By Procedure: Replacement Procedures; Repair Procedures
3) By Indication: Atrial Septal Defect; Patent Foramen Ovale; Ventricular Septal Defect; Aortic Valve Stenosis; Other Indications
4) By Age Group: Pediatric; Adults
Subsegments:
1) By Heart Valve Devices: Transcatheter Heart Valves; Surgical Heart Valves; Annuloplasty Rings2) By Annuloplasty Rings: Mitral Annuloplasty Rings; Aortic Annuloplasty Rings
3) By Occluders: Atrial Septal Defect (Asd) Occluders; Patent Foramen Ovale (Pfo) Occluders
4) By Delivery Systems: Balloon Delivery Systems; Catheter-Based Delivery Systems
Key Companies Mentioned: Abbott Laboratories; Medtronic Plc; Boston Scientific Corporation; Terumo Corporation; Edwards Lifesciences Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Structural Heart Devices market report include:- Abbott Laboratories
- Medtronic Plc
- Boston Scientific Corporation
- Terumo Corporation
- Edwards Lifesciences Corporation
- W. L. Gore & Associates Inc.
- Bracco Group
- LivaNova PLC
- Meril Life Sciences Pvt. Ltd.
- CryoLife Inc.
- Braile Biomédica
- TTK Healthcare Limited
- Direct Flow Medical Inc.
- JenaValve Technology Inc.
- Micro Interventional Devices Inc.
- CardioKinetix Inc.
- Xeltis AG
- Ancora Heart Inc.
- JOMDD Inc.
- Valcare Medical
- BioStable Science & Engineering Inc.
- Comed B.V.
- Transcatheter Technologies GmbH
- Navilyst Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 11.44 Billion |
| Forecasted Market Value ( USD | $ 16.48 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |