Key Takeaways
- In the United States, ruptured abdominal aortic aneurysms are responsible for an estimated 15,000 deaths annually, ranking as the 13th leading cause of death in the region.
- In April 2023, Medtronic plc, declared positive results from a 10-year post-market ENGAGE registry for its Endurant stent graft system which provides endovascular aneurysm repair (EVAR) to treat the abdominal aortic aneurysm.
- In December 2023, W. L. Gore & Associates, Inc. announced the first patient treatment with Gore ascending stent graft in the ARISE II study to treat pathologies involving the ascending aorta, marking an advancement in minimally invasive procedures.
The market is witnessing increased investment in research and development activities by the key market players in order to meet the growing demand for improved and safer aortic stent grafts. In April 2023, Medtronic plc, an American medical device company, announced positive results from a 10-year post-market ENGAGE registry for its Endurant stent graft system which provides endovascular aneurysm repair (EVAR) to treat abdominal aortic aneurysm. The data was presented at the 2023 Charing Cross Symposium in London, revealing 94.7% of cases reporting freedom from aneurysm-related mortality out of a pool of 400 patients. The presence of such real-world patient evidence is likely to boost the trust of patients and healthcare providers in the efficacy of aortic stent grafts, thereby fuelling North America aortic stent graft market growth.
In December 2023, W. L. Gore & Associates, Inc., an American multinational material science company, announced the first patient treatment with Gore ascending stent graft to treat pathologies involving the ascending aorta. It was based on the results of ARISE II, a pivotal study by the United States Food and Drug Administration (FDA). The study marks an advancement in minimally invasive procedures by using endovascular repair rather than conventional open surgical repair. The development of such minimally invasive alternatives which can reduce recovery times as well as the risk of complications is likely to augment the North America aortic stent graft market size in the forecast period.
The market is also driven by the rising technological innovation in stent grafts, the increased accessibility to novel medical devices in healthcare settings, and the growing elderly population at a greater risk of developing aortic aneurysms. Moreover, the presence of an advanced infrastructure and heightened patient awareness in the region is expected to bolster the market growth in the forecast period.
North America Aortic Stent Graft Market Segmentation
“North America Aortic Stent Graft Market Report and Forecast 2024-2032” offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Abdominal Aortic Stent Grafts
- Thoracic Aortic stent Grafts
Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centers
- Others
Market Breakup by Countries
- United States of America
- Canada
North America Aortic Stent Graft Market: Competitor Landscape
The key features of the market report include patent analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Cook Medical, Inc.
- W.L. Gore & Associates
- MicroPort Scientific Corporation Inc.
- Medtronic Plc.
- Lombard Medical, Inc.
- Endologix, Inc.
- Terumo Corporation Inc.
- Cardinal Health Inc.
- Becton, Dickinson and Company
- Cryolife Inc.
FAQs
What was the North America aortic stent graft market value in 2023?The market attained a value of D 1.3 billion in 2023 driven by the growing geriatric population along with the advancements in stent graft materials and design in the region.
What is the North America aortic stent graft market forecast outlook for 2024-2032?
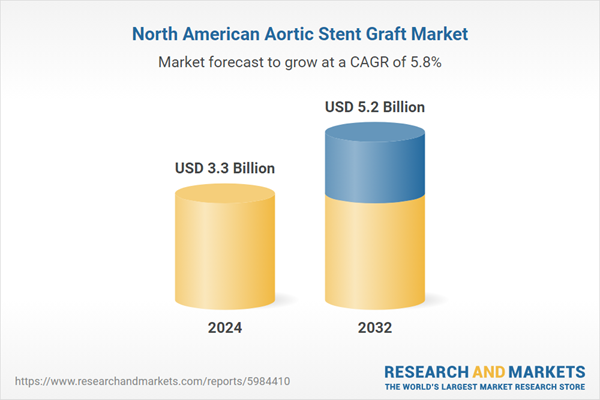
The market is anticipated to grow at a CAGR of 10.59% during the forecast period of 2024-2032 and estimated to reach a value of USD 3.3 billion by 2032.
What are the major factors aiding the North America aortic stent graft market demand?
The growing demand for minimally invasive procedures and rising healthcare expenditure are fuelling the demand for the regional market.
What are the major North America aortic stent graft market trends?
One of the significant trends in the market is the increased number of clinical studies to evaluate aortic stent graft efficacy and safety profile. In April 2023, Medtronic plc, announced positive results for its Endurant stent graft system which provides endovascular aneurysm repair (EVAR) to treat abdominal aortic aneurysm.
What is the market segmentation based on the product?
Based on the product, the market is segmented into abdominal aortic stent grafts and thoracic aortic stent grafts.
What are the major end users of aortic stent grafts in North America?
Major end users of the market include hospitals, and ambulatory surgical centres, among others.
What is the market segmentation by countries?
The market segmentation by countries includes the United States of America and Canada.
Who are the key players involved in the North America aortic stent graft market?
The key market players are Cook Medical, Inc., W.L. Gore & Associates, MicroPort Scientific Corporation Inc., Medtronic PLC, Lombard Medical, Inc., Endologix, Inc., Terumo Corporation Inc., Cardinal Health Inc., Becton, Dickinson and Company, and CryoLife Inc.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Cook Medical Inc.
- W.l. Gore & Associates
- MicroPort Scientific Corporation Inc.
- Medtronic Plc.
- Lombard Medical Inc.
- Endologix
- INC
- Terumo Corporation Inc.
- Cardinal Health Inc.
- Becton
- Dickinson and Company Cryolife Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | June 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 5.2 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | North America |
| No. of Companies Mentioned | 11 |