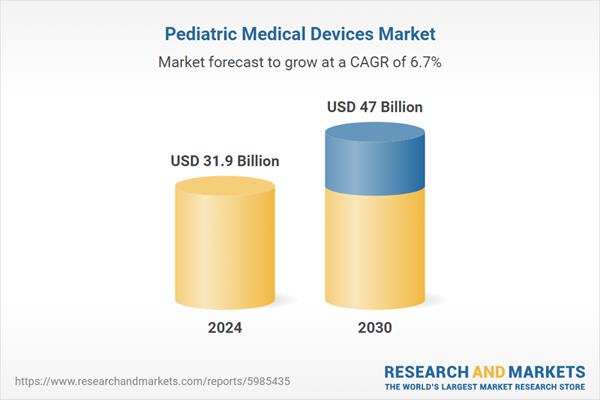
Global Pediatric Medical Devices Market - Key Trends and Drivers Summarized
Pediatric medical devices are essential tools designed to diagnose, treat, and manage health conditions in children, from newborns to adolescents up to 21 years old. These devices include a broad range of equipment, from simple monitors to complex surgical implements specifically engineered to address the physiological and anatomical needs of growing bodies. The design and production of such devices are heavily regulated under the FD&C Act to ensure safety and effectiveness, tailored to the unique requirements of different pediatric subpopulations: neonates, infants, children, and adolescents. There's a notable challenge in developing these devices due to the smaller market size and the high standards required for safety and efficacy. These challenges, along with the need for devices that accommodate rapid growth and activity levels of children, drive the demand for innovative solutions in pediatric healthcare technology.The development and enhancement of pediatric medical devices are fueled by both necessity and legislative support, such as the Pediatric Medical Device Safety and Improvement Act (PMDSIA) and initiatives like the Pediatric Device Consortia (PDC) grant program. These initiatives aim to encourage the design and market introduction of pediatric-specific medical solutions by providing funding, regulatory support, and expert advice to manufacturers. Advances in technology such as 3D printing have begun to play a pivotal role, enabling the customization of devices to individual anatomical requirements and the rapid prototyping of new device designs. This technology not only allows for the creation of devices that grow with the child but also enhances the functionality and longevity of these devices. Additionally, real-world data (RWD) is increasingly being utilized to gather evidence from routine clinical care, helping overcome the lack of clinical trial data for pediatric populations and facilitating the development of devices that are both safe and effective for young patients.
The growth in the pediatric medical device market is driven by several factors, highlighting a shift towards more tailored, efficient, and effective treatments for younger patients. Technological advancements in device miniaturization allow for the development of smaller, less invasive devices specifically designed for children's unique needs. There is also a growing focus on pediatric-specific research and innovation spurred by an increased incidence of chronic diseases among children, which necessitates ongoing monitoring and treatment. Parental demand for advanced treatments and the integration of digital health technologies are also significant growth drivers. Additionally, regulatory initiatives that facilitate faster approval and market entry for pediatric devices, along with educational initiatives aimed at healthcare providers, contribute significantly to the expansion of this sector. These factors collectively enhance the adoption and development of pediatric medical devices, ensuring that young patients receive the most advanced and appropriate care possible.
Report Scope
The report analyzes the Pediatric Medical Devices market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Device (In Vitro Diagnostic (IVD) Devices, Anesthesia & Respiratory Care Devices, Neonatal ICU Devices, Cardiology Devices, Diagnostic Imaging Devices, Other Devices); End-Use (Hospitals End-Use, Pediatric Clinics End-Use, Ambulatory Surgery Centers (ASCs) End-Use, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the In Vitro Diagnostic (IVD) Devices segment, which is expected to reach US$12.3 Billion by 2030 with a CAGR of a 7.6%. The Anesthesia & Respiratory Care Devices segment is also set to grow at 7.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $8.6 Billion in 2024, and China, forecasted to grow at an impressive 10.7% CAGR to reach $10.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pediatric Medical Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pediatric Medical Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pediatric Medical Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Inc., Atom Medical Corporation, Boston Scientific Corporation, Cardinal Health, Elektro-Mag Laboratuvar Aletleri Sanayi Ve Ticaret Anonim Sirketi (Elektro-Mag) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 45 companies featured in this Pediatric Medical Devices market report include:
- Abbott Laboratories, Inc.
- Atom Medical Corporation
- Boston Scientific Corporation
- Cardinal Health
- Elektro-Mag Laboratuvar Aletleri Sanayi Ve Ticaret Anonim Sirketi (Elektro-Mag)
- Fritz Stephan GmbH
- GE Healthcare
- Hamilton Medical AG
- Laborie Medical Technologies Corp.
- Medtronic
- Ningbo David Medical Device Co. Ltd
- Siemens Medical Solutions USA, Inc.
- Stryker Corporation
- Trimpeks Ith. Ihr. Tur. ve Tic. A.S. (Vivocare)
- TSE MEDICAL
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories, Inc.
- Atom Medical Corporation
- Boston Scientific Corporation
- Cardinal Health
- Elektro-Mag Laboratuvar Aletleri Sanayi Ve Ticaret Anonim Sirketi (Elektro-Mag)
- Fritz Stephan GmbH
- GE Healthcare
- Hamilton Medical AG
- Laborie Medical Technologies Corp.
- Medtronic
- Ningbo David Medical Device Co. Ltd
- Siemens Medical Solutions USA, Inc.
- Stryker Corporation
- Trimpeks Ith. Ihr. Tur. ve Tic. A.S. (Vivocare)
- TSE MEDICAL
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 294 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 31.9 Billion |
| Forecasted Market Value ( USD | $ 47 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |