Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Advancements in Biotechnology
Continuous innovations in biotechnology have revolutionized cell isolation techniques, marking a pivotal advancement in biomedical research and clinical applications. Key technologies such as magnetic-activated cell sorting (MACS), fluorescence-activated cell sorting (FACS), and microfluidic-based systems have emerged as cornerstones in the field, offering unprecedented precision and efficiency in isolating specific cell types. MACS utilizes magnetic nanoparticles conjugated with antibodies to selectively bind and separate target cells from heterogeneous cell populations. This technique enables gentle isolation of cells based on surface markers, preserving cell viability and functionality, which is crucial for downstream applications in stem cell research, cancer biology, and immunology. In January 2022, Sony Corporation unveiled its latest innovation, the CGX10 Cell Isolation System, designed to rapidly and efficiently sort cells with exceptional purity within a closed environment. The CGX10 is engineered to conduct cell sorting operations while maintaining a sterile state, ensuring the integrity of cell analysis and isolation processes. This capability is particularly crucial in advanced fields like cell-based immunotherapy, which is gaining prominence as a treatment for conditions such as cancer and autoimmune diseases. The growing demand for cell therapies in these medical applications necessitates precise cell isolation techniques that deliver high purity and viability. Sony's CGX10 addresses this need by providing a closed system that enhances the safety and reliability of cell isolation procedures, thereby supporting the development and production of innovative cell therapies.Similarly, FACS leverages laser-based detection and sorting of cells tagged with fluorescent labels, allowing rapid and high-throughput isolation of cell populations based on multiple parameters such as size, shape, and fluorescence intensity. This technology offers unparalleled accuracy in separating rare cell subsets and has become indispensable in immunophenotyping and antibody discovery. Microfluidic-based systems represent another innovation, leveraging precise control of fluid flow within microscale channels to isolate cells based on physical and biochemical properties. These systems integrate miniaturized platforms with automated processes, enabling efficient isolation of cells with minimal sample volumes and reduced processing times. They are increasingly used in point-of-care diagnostics, circulating tumor cell analysis, and single-cell genomics. The continuous enhancement of these technologies not only improves the efficiency and scalability of cell isolation processes but also expands the scope of applications across various disciplines. Researchers and clinicians benefit from enhanced capabilities to isolate rare cell populations, investigate disease mechanisms at the cellular level, and develop targeted therapies tailored to individual patient profiles.
Rising Demand in Regenerative Medicine
The expanding use of cell isolation techniques in regenerative medicine, especially in the realms of stem cell research and therapy, is a significant driver propelling market growth. These techniques play a pivotal role in the extraction of pure and viable stem cell populations essential for various therapeutic applications. Stem cells hold immense promise in regenerative medicine due to their unique ability to differentiate into specialized cell types and replenish damaged tissues. Cell isolation methods are critical in separating and purifying these stem cells from heterogeneous sources such as bone marrow, adipose tissue, and umbilical cord blood. By isolating specific subsets of stem cells with desired characteristics - whether it's pluripotency, multipotency, or specific lineage commitment - researchers and clinicians can harness their therapeutic potential more effectively.In stem cell therapy, isolated cells can be used to repair or replace damaged tissues and organs, offering hope for treating degenerative diseases, injuries, and congenital disorders. The purity and viability of isolated stem cells are crucial factors determining the success and safety of these therapies. Advancements in cell isolation technologies, such as magnetic-activated cell sorting (MACS), fluorescence-activated cell sorting (FACS), and microfluidic-based systems, have significantly enhanced the efficiency and accuracy of isolating stem cells. These technologies enable researchers to isolate rare and specific cell populations with high precision, ensuring that therapeutic interventions are tailored to individual patient needs.
Biopharmaceutical Research and Development
The pharmaceutical industry's relentless pursuit of biopharmaceuticals, encompassing monoclonal antibodies and cell-based therapies, hinges critically on advanced cell isolation technologies. These technologies serve as foundational tools essential for the isolation and characterization of specific cell types pivotal in the development and production of innovative therapeutics.Monoclonal antibodies (mAbs), for instance, are engineered to target specific antigens on cells and are widely used in treating various diseases, including cancers and autoimmune disorders. Cell isolation techniques play a crucial role in producing monoclonal antibodies by isolating hybridoma cells that secrete these antibodies. Techniques like fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS) are employed to isolate and purify these cells, ensuring high specificity and potency of the resulting therapeutic antibodies. Similarly, the advent of cell-based therapies, such as chimeric antigen receptor (CAR) T-cell therapies and stem cell therapies, underscores the need for precise cell isolation methods. CAR T-cell therapies involve genetically modifying a patient's T-cells to recognize and attack cancer cells, necessitating the isolation of patient-derived T-cells with high purity and functionality. Advanced cell isolation technologies ensure the isolation of T-cells capable of robust anti-tumor activity, thereby enhancing the therapeutic efficacy and safety of these treatments.
Increasing Incidence of Chronic Diseases
The increasing prevalence of chronic diseases, encompassing cancer, cardiovascular disorders, and autoimmune conditions, underscores the critical need for advanced cell isolation techniques in biomedical research and clinical practice. These techniques play a pivotal role in facilitating the study of disease mechanisms, the development of targeted therapies, and ultimately, in enhancing patient outcomes.Cancer, as a leading cause of morbidity and mortality globally, benefits significantly from advancements in cell isolation technologies. By the year 2050, it is projected that the prevalence of cancer in Japan will reach approximately 3,665,900 cases (ranging from 3,210,200 to 4,201,400), reflecting a 13.1% increase from the figures recorded in 2020. This anticipated rise is largely attributed to a substantial increase in the number of female cancer survivors, showing a significant growth rate of 27.6%. In contrast, the increase among male survivors is more modest at 0.8%. As a result, females are expected to surpass males in terms of cancer prevalence starting from the year 2040 onwards. Researchers rely on these techniques to isolate cancer cells from patient samples, enabling detailed molecular and genetic analysis. By studying the characteristics and behavior of isolated cancer cells, scientists gain insights into disease progression, drug resistance mechanisms, and potential therapeutic targets. This knowledge informs the development of personalized cancer therapies aimed at improving treatment efficacy and reducing adverse effects. In cardiovascular disorders, such as heart disease and stroke, cell isolation techniques are instrumental in studying the pathophysiology of the disease. Isolation of specific cell types, such as endothelial cells or cardiomyocytes, allows researchers to investigate cellular dysfunction, inflammatory processes, and tissue regeneration mechanisms. These insights contribute to the development of novel treatments, including cell-based therapies designed to repair damaged heart tissue and restore cardiac function.
Key Market Challenges
Regulatory Hurdles and Compliance
The Japan Cell Isolation Market operates within a regulatory framework overseen by the Pharmaceuticals and Medical Devices Agency (PMDA), which imposes stringent requirements for the approval of new cell isolation technologies. To bring innovative products to market, companies must undergo rigorous preclinical and clinical evaluations aimed at demonstrating safety, efficacy, and adherence to high-quality standards. Obtaining regulatory approval involves navigating a complex landscape characterized by stringent guidelines and lengthy approval processes. These processes are designed to safeguard patient safety and ensure the reliability of new technologies in clinical settings. Companies face significant challenges in meeting these regulatory demands, as compliance requires meticulous documentation, robust data from preclinical and clinical studies, and adherence to Good Manufacturing Practice (GMP) standards.The prolonged approval timelines associated with regulatory reviews can pose substantial barriers to market entry and product commercialization. Delays in obtaining regulatory clearance not only hinder innovation but also increase the time-to-market for new cell isolation technologies. This can limit companies' ability to capitalize on early-mover advantages and respond swiftly to market demands. The evolving nature of regulatory guidelines necessitates continuous adaptation and investment in compliance efforts. Companies must allocate substantial resources, both financial and human, to navigate regulatory complexities effectively. This includes maintaining close communication with regulatory authorities, conducting comprehensive risk assessments, and implementing corrective measures to address regulatory feedback and ensure alignment with updated guidelines.
High Cost of Development and Commercialization
Developing and bringing new cell isolation technologies to market in Japan requires substantial investments across various facets of the business, including research and development (R&D), clinical trials, manufacturing infrastructure, and regulatory compliance. These investments are essential to validate the safety, efficacy, and commercial viability of innovative products in the competitive biotechnology landscape. Research and development constitute a significant portion of the upfront costs, as companies invest in discovering and refining novel cell isolation techniques. This phase involves conducting exploratory research, optimizing technologies, and conducting preliminary testing to establish proof of concept. R&D efforts also encompass scaling up production processes and refining manufacturing protocols to ensure consistent product quality and reliability.Clinical trials represent another critical phase in the development pathway, where companies must conduct rigorous testing to evaluate the safety and efficacy of new cell isolation technologies in human subjects. These trials are designed to generate robust clinical data that support regulatory submissions and provide evidence of therapeutic benefits. The costs associated with planning, executing, and analyzing clinical trials can be substantial, requiring meticulous adherence to Good Clinical Practice (GCP) guidelines and ethical considerations. Investments in manufacturing infrastructure are essential to support scalable production of cell isolation technologies once regulatory approval is secured. Establishing manufacturing facilities equipped with state-of-the-art equipment and technologies ensures efficient production processes and compliance with Good Manufacturing Practice (GMP) standards. This infrastructure investment is crucial for meeting market demand, maintaining product supply continuity, and achieving economies of scale to enhance cost-effectiveness.
Key Market Trends
Growing Academic and Research Collaborations
Collaborative efforts between academic institutions, research organizations, and biotechnology companies play a crucial role in advancing cell isolation technologies and driving market growth. These collaborations foster a dynamic environment where knowledge exchange, interdisciplinary research, and innovation thrive, ultimately leading to the development of novel techniques and applications in cell isolation.Academic institutions serve as hubs of scientific inquiry, conducting fundamental research into cellular biology, disease mechanisms, and technological advancements. Researchers at universities and research organizations explore new methodologies for isolating specific cell types, improving existing techniques, and validating their efficacy through rigorous experimentation. These efforts contribute essential insights into the nuances of cellular behavior and the development of disease states. Biotechnology companies, on the other hand, specialize in translating scientific discoveries into practical applications and commercial products. By partnering with academic and research institutions, these companies gain access to cutting-edge research and intellectual capital. Collaborations enable biotechnology firms to integrate innovative cell isolation technologies into their product pipelines, enhancing the efficiency, scalability, and market competitiveness of their offerings.
Expansion of Cell Therapy Applications
The expanding landscape of cell therapies, including groundbreaking advancements in chimeric antigen receptor (CAR) T-cell therapies and mesenchymal stem cell treatments, underscores the critical importance of efficient cell isolation techniques in biomedical research and clinical applications. These advanced therapies harness the therapeutic potential of specific cell populations to combat diseases ranging from cancer to autoimmune disorders, driving significant growth in the cell isolation market.CAR T-cell therapies, for instance, involve genetically modifying a patient's T-cells to express CARs, enabling them to recognize and target cancer cells with precision. The success of CAR T-cell therapies hinges on the ability to isolate and expand patient-derived T-cells efficiently while preserving their functionality and purity. Cell isolation technologies such as magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) play a pivotal role in isolating T-cells from a patient's blood or bone marrow, enriching them for CAR modification, and subsequently reinfusing them into the patient for therapeutic effect.
Segmental Insights
Product Insights
Based on the Product, consumables play a pivotal role in driving the Japan Cell Isolation Market, primarily due to their essential function in facilitating efficient and reliable cell isolation processes. These consumables encompass a wide array of critical materials and supplies necessary for isolating, purifying, and maintaining cell populations in laboratory and clinical environments. Reagents, kits, culture media, and disposable labware are among the key consumables that are integral to various cell isolation techniques, including magnetic-activated cell sorting (MACS), fluorescence-activated cell sorting (FACS), and microfluidic-based systems.The dominance of consumables in the market is underscored by their high frequency of use and necessity for ongoing research, diagnostics, and therapeutic applications. Researchers, clinicians, and biotechnologists rely heavily on these consumables for conducting routine and specialized experiments, ensuring consistent workflow and experimental reliability. Consumables are tailored to specific cell types, markers, and research objectives, allowing for customization that optimizes outcomes in diverse biomedical contexts. Technological advancements continually enhance the performance and functionality of consumables, supporting their integration into automated systems and high-throughput workflows. This evolution not only improves laboratory efficiency and productivity but also expands the utility of consumables across the burgeoning biotechnology sector in Japan.
Cell Type Insights
Based on Cell Type, human cells significantly dominate over animal cells due to their critical importance in biomedical research, clinical applications, and therapeutic advancements. Human cells are fundamental to understanding human biology, disease mechanisms, and developing targeted therapies tailored to individual patient needs. One of the primary reasons for the dominance of human cells is their direct relevance to human health. Researchers and clinicians extensively utilize human cells to study diseases such as cancer, cardiovascular disorders, and neurological conditions. The ability to isolate and analyze specific human cell types enables researchers to unravel complex disease pathways, identify biomarkers, and develop innovative treatments. For instance, stem cells isolated from human tissues offer promising avenues for regenerative medicine, where they can potentially repair damaged tissues and organs.Advancements in personalized medicine underscore the significance of human cells in treatment strategies. Cell-based therapies, such as CAR T-cell therapy for cancer and mesenchymal stem cell therapy for immune disorders, rely exclusively on human cells due to their compatibility with the human immune system and reduced risk of immune rejection. These therapies demonstrate the therapeutic potential of human cells in treating previously untreatable conditions and improving patient outcomes. Ethical considerations also contribute to the preference for human cells in research and clinical settings. While animal cells are valuable for certain types of research and preclinical studies, the use of human cells is prioritized to better reflect human physiology and responses to treatments. This ethical stance aligns with regulatory guidelines and patient-centric approaches in healthcare, ensuring that research outcomes translate more effectively into clinical benefits.
Regional Insights
Kanto hosts a dense concentration of prestigious universities and research institutions that are pivotal in advancing biotechnological research, including cell isolation techniques. Institutions such as the University of Tokyo, Tokyo Institute of Technology, and Keio University are at the forefront of scientific research and innovation. These universities have well-established departments and laboratories focused on cell biology, regenerative medicine, and biotechnology, where researchers actively develop and refine cell isolation methods. Their proximity fosters collaboration among academia, government research agencies, and private industry, facilitating knowledge exchange and technology transfer.Tokyo serves as a hub for major biotechnology companies and pharmaceutical firms that drive innovation in cell isolation technologies. Companies like Astellas Pharma, Daiichi Sankyo, and Takeda Pharmaceutical are headquartered in Tokyo or have significant research facilities in the region. These companies invest heavily in R&D to develop novel therapies, many of which require advanced cell isolation techniques for drug discovery, development, and clinical trials. Tokyo's status as Japan's political and economic center attracts international collaborations and partnerships in biotechnology. The city hosts numerous biotechnology conferences, symposiums, and workshops where researchers and industry professionals gather to discuss the latest advancements in cell isolation and other biotechnological innovations. These interactions contribute to the dissemination of knowledge and the adoption of cutting-edge technologies in the field.
Key Market Players
- Becton Dickinson Japan Co., Ltd.
- Merck Biopharma Co., Ltd.
- Terumo BCT Japan, Inc.
- GenScript Japan Co., Ltd.
- Sony Corporation
Report Scope:
In this report, the Japan Cell Isolation Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Japan Cell Isolation Market, By Product:
- Consumables
- Instruments
Japan Cell Isolation Market, By Cell Type:
- Human Cells
- Animal Cells
Japan Cell Isolation Market, By Source:
- Bone Marrow
- Cord Blood/Embryonic Stem Cells
- Adipose Tissue
Japan Cell Isolation Market, By Technique:
- Centrifugation-Based Cell Isolation
- Surface Marker-Based Cell Isolation
- Filtration-Based Cell Isolation
Japan Cell Isolation Market, By Application:
- Biomolecule Isolation
- Cancer Research
- Stem Cell Research
- In Vitro Diagnostics
- Others
Japan Cell Isolation Market, By End User:
- Biotechnology and Biopharmaceutical Companies
- Research Laboratories and Institutes
- Hospitals and Diagnostic Laboratories
- Cell Banks
Japan Cell Isolation Market, By Region:
- Hokkaido
- Tohoku
- Kanto
- Chubu
- Kansai
- Chugoku
- Shikoku
- Kyushu
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Japan Cell Isolation Market.Available Customizations:
Japan Cell Isolation Market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Becton Dickinson Japan Co., Ltd.
- Merck Biopharma Co., Ltd.
- Terumo BCT Japan, Inc.
- GenScript Japan Co., Ltd.
- Sony Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 80 |
| Published | July 2024 |
| Forecast Period | 2024 - 2030 |
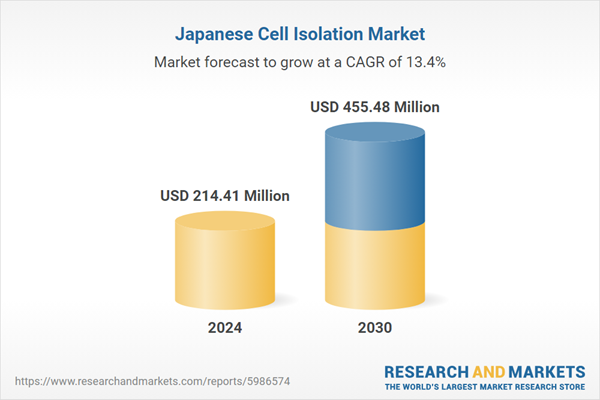
| Estimated Market Value ( USD | $ 214.41 Million |
| Forecasted Market Value ( USD | $ 455.48 Million |
| Compound Annual Growth Rate | 13.3% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 5 |