Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Increasing Diabetes Prevalence
Diabetes prevalence in Japan has been steadily increasing, influenced by a combination of demographic shifts and lifestyle changes. The country's aging population, characterized by longer life expectancy and a higher incidence of chronic diseases, including diabetes, has placed significant strain on healthcare resources. According to International Diabetes Federation, Japan is a prominent member of the International Diabetes Federation's (IDF) Western Pacific region, which encompasses 20 countries and territories. Globally, diabetes affects a staggering 537 million people, with 206 million of these individuals residing in the Western Pacific Region alone. Projections indicate that by the year 2045, the number of people living with diabetes in this region is expected to escalate significantly to reach approximately 260 million. These statistics underscore the urgent need for proactive measures and innovative strategies to address the growing burden of diabetes in Japan and across the Western Pacific Region. Efforts focusing on prevention, early detection, and effective management are crucial to mitigating the impact of diabetes on public health and ensuring better quality of life for individuals affected by this chronic condition.Urbanization has also played a role, fostering sedentary lifestyles and unhealthy dietary habits that contribute to the onset of diabetes. Recent statistics highlight a rising trend in diabetes among Japanese adults, necessitating more effective management strategies. This escalating prevalence underscores the critical need for continuous glucose monitoring devices that can provide accurate and timely data to help individuals manage their blood glucose levels effectively. In response to this demographic shift and healthcare challenge, there is a growing demand for advanced glucose monitoring solutions that can integrate seamlessly into Japan's healthcare system, supporting better disease management and improving overall health outcomes.
Increasing Innovations
The glucose monitoring devices market in Japan thrives on a continual wave of technological progress that has significantly transformed diabetes management. One of the groundbreaking innovations in this field is the development of continuous glucose monitors (CGMs). Unlike traditional methods that require frequent finger pricks to measure blood glucose levels, CGMs offer real-time monitoring through a sensor inserted under the skin. This continuous monitoring capability provides users with a steady stream of glucose data throughout the day and night, enabling them to make timely adjustments to their diet, medication, and activities. Terumo Corporation announced that effective December 1, 2022, the Dexcom G6 CGM System will now benefit from expanded reimbursement coverage under the Japanese medical insurance system. The inclusion of the new "C150" category means a broader range of individuals with diabetes in Japan will now have access to reimbursement for using the Dexcom G6 CGM System.CGMs have revolutionized diabetes care by enhancing both convenience and compliance among patients. The elimination of frequent finger pricks reduces discomfort and improves overall quality of life. The accuracy of CGMs in monitoring glucose levels is superior, offering more precise insights into blood sugar trends and fluctuations. This real-time data is crucial for patients and healthcare providers alike, enabling informed decision-making and proactive management of diabetes. These technological advancements extend beyond the devices themselves. CGMs and other advanced glucose monitoring technologies integrate seamlessly with digital health platforms and mobile apps. This integration allows for continuous data collection, analysis, and sharing between patients and healthcare providers. Clinicians can remotely monitor their patients' glucose levels and adjust treatment plans accordingly, leading to more personalized and effective care strategies.
Government Initiatives
The Japanese government has proactively responded to the increasing prevalence of diabetes by implementing a series of strategic initiatives aimed at improving healthcare outcomes and addressing the challenges posed by this chronic condition. Central to these efforts are policies designed to enhance diabetes management and strengthen healthcare infrastructure across the country. Recognizing the importance of advanced glucose monitoring devices in effective diabetes care, the government has introduced subsidies and incentives to encourage both healthcare providers and patients to adopt these technologies. These financial incentives make cutting-edge monitoring devices more accessible and affordable, thereby facilitating their widespread adoption in clinical settings and among individuals managing diabetes at home. By reducing financial barriers, the government aims to improve overall diabetes management outcomes and mitigate the long-term health complications associated with the disease.Japan's regulatory frameworks play a crucial role in facilitating the introduction and adoption of innovative glucose monitoring devices. Stringent safety and efficacy standards ensure that new technologies meet rigorous criteria for performance and reliability. This regulatory environment not only safeguards patient health but also instills confidence in healthcare providers and patients regarding the effectiveness and safety of these devices. The government's supportive stance creates favorable market conditions for manufacturers and developers of glucose monitoring technologies. This encouragement fosters a conducive environment for research and development, innovation, and market growth. As a result, companies are incentivized to invest in new technologies that can further enhance the accuracy, usability, and integration capabilities of glucose monitoring devices.
Healthcare Expenditure
In Japan, the escalating healthcare expenditure, combined with a growing emphasis on preventive healthcare, has catalyzed significant investments in technologies aimed at managing chronic conditions like diabetes. Healthcare providers are increasingly prioritizing cost-effective solutions that not only enhance patient outcomes but also mitigate the long-term financial burden associated with diabetes-related complications. Glucose monitoring devices stand out as pivotal tools in this landscape, offering continuous monitoring capabilities that enable early detection of blood glucose fluctuations and proactive management of the condition.By facilitating timely intervention and adjustment of treatment plans, these devices help prevent severe complications such as diabetic ketoacidosis and hypoglycemia, which can lead to hospitalizations and increased healthcare costs. Continuous glucose monitoring supports personalized medicine approaches, allowing healthcare providers to tailor treatment strategies based on real-time data insights. This targeted approach not only improves patient adherence to therapy but also optimizes healthcare resource allocation. Investments in advanced monitoring technologies align closely with healthcare cost containment strategies in Japan. By reducing the incidence of acute diabetes-related complications, these technologies contribute to overall healthcare efficiency and sustainability. The adoption of innovative glucose monitoring solutions supports the broader goal of enhancing patient care quality and promoting better health outcomes across the population.
Key Market Challenges
Regulatory Hurdles and Approval Processes
The Japan Glucose Monitoring Devices Market faces significant challenges related to regulatory hurdles and approval processes. Japan has stringent regulatory requirements for medical devices, including glucose monitoring devices, which must undergo rigorous evaluations and obtain approvals from the Pharmaceuticals and Medical Devices Agency (PMDA). These regulatory processes often involve extensive documentation, clinical trials, and adherence to specific standards, which can be time-consuming and costly for manufacturers.One of the primary challenges is navigating the PMDA's approval process, which requires comprehensive data demonstrating the device's safety, efficacy, and reliability. The regulatory landscape in Japan is constantly evolving, with updates and amendments to existing regulations that manufacturers must continuously monitor and comply with. This dynamic regulatory environment poses challenges for companies aiming to introduce new glucose monitoring technologies quickly and efficiently to the market. Another aspect of the regulatory challenge is harmonizing Japan's requirements with international standards. Differences in regulatory expectations between Japan and other major markets, such as the EU or the US, require manufacturers to tailor their clinical trial strategies and data submissions specifically for the Japanese market. This can lead to additional complexities and delays in obtaining approvals, particularly for companies seeking simultaneous global market access.
Competitive Market Dynamics
The Japan Glucose Monitoring Devices Market is characterized by intense competition among both domestic and international manufacturers. This competitive landscape poses several challenges for companies aiming to establish or expand their presence in the market. Domestic manufacturers benefit from strong brand recognition and established distribution networks, while international players bring innovative technologies and global expertise. One of the primary challenges in this competitive environment is differentiation. Manufacturers must continually innovate and differentiate their products to capture market share. This includes developing glucose monitoring devices with advanced features such as real-time data analysis, integration with mobile applications, and enhanced user interface design. However, introducing innovative technologies requires substantial investments in research and development, which may pose financial challenges, particularly for smaller companies.Pricing pressures in the competitive market can impact profitability and market penetration. Healthcare reimbursement policies in Japan often prioritize cost-effectiveness and may impose pricing constraints on medical devices, including glucose monitoring devices. Manufacturers must navigate these pricing dynamics while maintaining product quality and ensuring profitability, which can be particularly challenging in a market with diverse consumer preferences and healthcare provider requirements. Another aspect of competitive dynamics is the need for effective marketing and distribution strategies. Companies must establish strong relationships with healthcare providers, hospitals, and pharmacies to ensure broad market access and effective product adoption. This requires navigating complex distribution channels and understanding regional variations in healthcare practices and patient preferences across different prefectures in Japan.
Key Market Trends
Technological Integration
The integration of glucose monitoring devices with smartphone apps, wearable technology, and digital health platforms has ushered in a new era of diabetes management in Japan, revolutionizing how patients and healthcare providers interact with and manage the disease. This technological synergy enables seamless connectivity between glucose monitoring devices and various digital platforms, facilitating real-time monitoring of blood glucose levels. Patients can conveniently track their glucose readings, trends, and patterns directly on their smartphones or wearable devices, providing them with immediate access to critical health information.This integration allows for effortless sharing of glucose data with healthcare providers, even remotely. Healthcare teams can remotely monitor their patients' glucose levels and receive alerts for any concerning trends, enabling timely interventions and adjustments to treatment plans. This proactive approach helps prevent complications and optimize diabetes management strategies tailored to each patient's individual needs. The accessibility and usability of integrated glucose monitoring solutions have significantly enhanced patient engagement and adherence to treatment regimens. Patients are empowered with greater insight into their health metrics, fostering a sense of control and ownership over their condition. The convenience of digital platforms encourages regular monitoring and facilitates communication between patients and healthcare providers, promoting collaborative decision-making and enhancing overall care quality.
Research and Development
Continued investment in research and development (R&D) by key market players significantly contributes to the advancement of glucose monitoring devices through innovation. These companies prioritize the development of next-generation technologies aimed at enhancing accuracy, reliability, and overall user experience. By leveraging substantial R&D investments, they introduce new products equipped with cutting-edge features such as predictive analytics and artificial intelligence algorithms. These innovations are tailored to meet the evolving needs and preferences of patients.The ongoing R&D efforts are crucial for obtaining regulatory approvals and conducting rigorous clinical validations. This ensures that the new glucose monitoring devices not only meet but exceed stringent safety and efficacy standards. Such adherence to regulatory requirements not only enhances patient trust but also strengthens market competitiveness and establishes differentiation among products. Overall, sustained R&D investments in glucose monitoring devices drive continuous innovation, leading to advanced technologies that promise superior performance, enhanced usability, and improved patient outcomes.
Segmental Insights
Product Type Insights
Based on the Product type, Continuous Glucose Monitoring (CGM) devices are increasingly dominating over traditional Self-Monitoring of Blood Glucose (SMBG) devices. This shift is driven by several factors that highlight the advantages of CGM technology in managing diabetes more effectively. Continuous Glucose Monitoring devices offer significant benefits compared to SMBG devices, particularly in terms of providing real-time and continuous glucose readings throughout the day and night. This continuous monitoring capability allows for a more comprehensive understanding of glucose trends, patterns, and fluctuations, which is crucial for optimizing diabetes management and making timely treatment adjustments. For patients with fluctuating glucose levels or those requiring tight glycemic control, CGM devices provide actionable insights that SMBG devices may not capture as comprehensively.Another key advantage of CGM devices is their ability to alert users to hypo- and hyperglycemic events in real-time. These alerts help patients and healthcare providers intervene promptly to prevent severe hypoglycemia or hyperglycemia episodes, thereby improving overall glycemic control and reducing the risk of diabetes-related complications. Such proactive management is particularly beneficial for patients with Type 1 diabetes, where maintaining stable glucose levels is critical for long-term health outcomes. CGM devices offer convenience and lifestyle benefits compared to traditional SMBG devices. Many CGM systems are designed for continuous wear, with sensors that can remain in place for several days, reducing the need for frequent fingerstick testing. This feature enhances patient compliance and reduces the burden associated with multiple daily glucose measurements, particularly for children, adolescents, and adults with busy lifestyles.
Application Insights
Based on Application, Type 2 Diabetes (T2D) is currently dominating over Type 1 Diabetes (T1D) and Gestational Diabetes Mellitus (GDM). This trend reflects the epidemiological profile and healthcare dynamics specific to Japan, where Type 2 Diabetes constitutes a significant portion of the diabetes burden. Type 2 Diabetes is characterized by insulin resistance and relative insulin deficiency, typically developing in adults but increasingly diagnosed in younger individuals due to lifestyle factors such as sedentary behavior, unhealthy diet, and obesity. In Japan, the prevalence of Type 2 Diabetes has been rising steadily, driven by aging demographics, urbanization, dietary changes, and genetic predispositions. This demographic shift has led to a larger patient population requiring ongoing glucose monitoring and management, thereby driving demand for glucose monitoring devices tailored to Type 2 Diabetes management. The management of Type 2 Diabetes often involves lifestyle modifications, oral medications, and in some cases, insulin therapy. Continuous Glucose Monitoring (CGM) devices, in particular, are increasingly favored for their ability to provide real-time glucose readings and facilitate personalized treatment adjustments aimed at achieving optimal glycemic control. This proactive approach to diabetes management aligns with current healthcare trends in Japan, where emphasis is placed on preventing diabetes-related complications through early detection and intervention.Regional Insights
Kanto stands out as the dominant region in terms of market size and healthcare influence. Kanto region, encompassing Tokyo and its surrounding prefectures such as Kanagawa, Chiba, and Saitama, is the most populous and economically vibrant region in Japan. Tokyo itself serves as the national capital and a major hub for healthcare innovation, research, and development. The concentration of leading hospitals, research institutions, medical universities, and pharmaceutical companies in Kanto fosters a robust ecosystem for healthcare technology, including glucose monitoring devices.Kanto region hosts a significant portion of Japan's population, including a large elderly demographic susceptible to diabetes and requiring continuous glucose monitoring and management. The prevalence of Type 2 Diabetes in urban areas like Tokyo is higher compared to rural regions, driven by lifestyle factors such as sedentary lifestyles, dietary habits, and stress levels associated with urban living. In terms of healthcare infrastructure, Kanto boasts advanced medical facilities equipped with state-of-the-art diagnostic tools and technologies, making it a preferred market for the adoption of advanced glucose monitoring devices such as Continuous Glucose Monitors (CGMs) and other innovative technologies. The region's healthcare providers are often early adopters of new medical technologies, driving demand and setting trends for diabetes management practices nationwide.
Key Market Players
- Terumo BCT Japan, Inc.
- ARKRAY, Inc.
- Nipro Corporation
- Abbott Japan Co., Ltd.
- Medtronic Japan Co., Ltd.
- B. Braun Aesculap Japan Co., Ltd.
- Provigate, Inc.
- Quantum Operation Inc.
- Hitachi, Ltd.
Report Scope:
In this report, the Japan Glucose Monitoring Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Japan Glucose Monitoring Devices Market, By Product Type:
- Self-Monitoring Glucose Devices
- Glucometers
- Test Strips
- Lancets
- Continuous Glucose Monitoring Devices
- Sensors
- Transmitters & Receivers
- Integrated Insulin Pumps
Japan Glucose Monitoring Devices Market, By Application:
- Type 1 Diabetes
- Type 2 Diabetes
- Gestational Diabetes
Japan Glucose Monitoring Devices Market, By End User:
- Home Care Settings
- Hospitals
- Others
Japan Glucose Monitoring Devices Market, By Region:
- Hokkaido
- Tohoku
- Kanto
- Chubu
- Kansai
- Chugoku
- Shikoku
- Kyushu
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Japan Glucose Monitoring Devices Market.Available Customizations:
Japan Glucose Monitoring Devices Market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Terumo BCT Japan, Inc.
- ARKRAY, Inc.
- Nipro Corporation
- Abbott Japan Co., Ltd.
- Medtronic Japan Co., Ltd.
- B. Braun Aesculap Japan Co., Ltd.
- Provigate, Inc.
- Quantum Operation Inc.
- Hitachi, Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 80 |
| Published | July 2024 |
| Forecast Period | 2024 - 2030 |
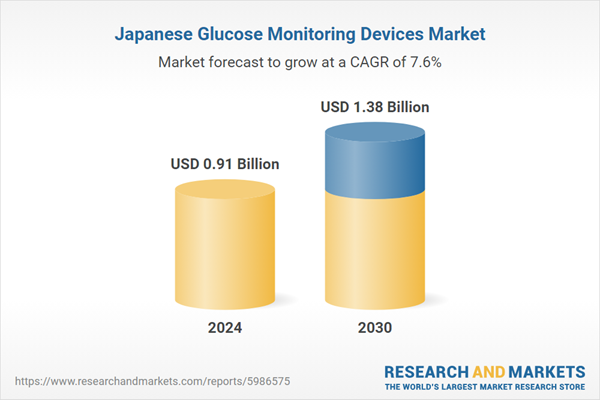
| Estimated Market Value ( USD | $ 0.91 Billion |
| Forecasted Market Value ( USD | $ 1.38 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 9 |