Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, a major obstacle impeding market progression is the rigorous regulatory approval process mandated for new diagnostic assays. Manufacturers face the task of navigating intricate compliance standards and enduring prolonged validation phases to prove clinical utility, which escalates development costs and postpones product launches. Additionally, uneven reimbursement coverage for emerging biomarkers across international healthcare systems can hinder commercial uptake and curtail the financial sustainability of testing solutions for newer providers.
Market Drivers
The rising global prevalence of cardiovascular diseases serves as a primary engine for the cardiac biomarkers market, generating consistent demand for diagnostic testing. With the incidence of conditions like heart failure and myocardial infarction increasing, medical providers are relying more heavily on sensitive biomarkers for effective risk stratification and rapid triage. This growing disease burden leads directly to elevated testing volumes in clinical and hospital environments, requiring a strong diagnostic infrastructure. According to the 'Vital Statistics Rapid Release' report by the Centers for Disease Control and Prevention in June 2025, heart disease-related deaths in the United States climbed to 683,037 in 2024, highlighting the essential need for precise tools to manage the expanding patient population and prevent severe outcomes.Concurrently, technological advancements in high-sensitivity biomarker assays are broadening market potential by improving diagnostic accuracy. These sophisticated solutions enable the detection of cardiac troponin at much lower concentrations, allowing for the identification of myocardial injury significantly earlier than traditional methods, which supports a shift toward proactive patient care. The market's favorable reception of these innovations is evident in financial metrics; according to Abbott's 'Third-Quarter 2024 Results' press release from October 2024, global Core Laboratory Diagnostics sales grew by 4.5 percent organically, fueled by the uptake of updated diagnostic systems. Underscoring this trend of high-volume usage, Roche reported delivering a total of 30 billion diagnostic tests globally during the preceding fiscal year in 2025.
Market Challenges
The exacting regulatory approval process acts as a significant hurdle that directly impedes the expansion of the Global Cardiac Biomarkers Market. Manufacturers are required to maneuver through complex compliance structures and undertake comprehensive validation stages to establish the clinical utility of new diagnostic assays. This intense scrutiny results in extended development schedules and substantially increased operational expenses before a product can reach the commercial stage. Consequently, the substantial investment of time and capital needed to obtain approvals frequently discourages companies from launching innovative biomarkers, thereby slowing the rate of innovation and limiting the introduction of advanced diagnostic solutions.The consequence of these regulatory pressures is visible in recent industry trends, where there is a strategic withdrawal from markets characterized by complicated compliance mandates. According to MedTech Europe in 2024, the preference for the European Union as the primary region for initial product launches by major in vitro diagnostic manufacturers fell by 40 percent due to difficulties linked to the new regulatory landscape. This data point emphasizes how burdensome regulatory requirements compel firms to reallocate resources and postpone launch strategies. By hindering the rollout of necessary diagnostic tools, these regulatory obstacles effectively limit revenue potential and restrict the sector's broader commercial growth.
Market Trends
The rise of Automated High-Throughput Immunoassay Systems is fundamentally transforming the Global Cardiac Biomarkers Market as clinical labs focus on workflow efficiency to handle growing testing loads. Manufacturers are increasingly releasing integrated platforms that merge immunodiagnostics with clinical chemistry, thereby minimizing manual tasks and accelerating turnaround times for essential cardiac assays. This strong demand for sophisticated diagnostic infrastructure is reflected in recent market performance; according to Roche's January 2025 press release titled 'Roche reports strong 2024 results with 7% sales growth,' the company's Diagnostics Division saw base business sales rise by 8 percent at constant exchange rates, driven by the continued adoption of immunodiagnostic solutions and the launch of the cobas Mass Spec system.Simultaneously, a movement toward Personalized Medicine and Value-Based Cardiac Care is shaping market dynamics, urging healthcare providers to implement diagnostic protocols that reduce unnecessary resource use. By utilizing high-precision biomarkers for swift risk stratification, hospitals can efficiently rule out acute cardiac events, which helps alleviate emergency department congestion and lowers overall healthcare expenses. This trend toward enhanced operational efficiency is backed by recent statistics; according to a GlobalRPH article from October 2025 titled 'High-Sensitivity Troponin Testing: The End of Unnecessary Chest Pain Admissions?', the adoption of high-sensitivity cardiac troponin I testing protocols led to an absolute reduction of 5.6 percent in hospital admissions related to chest pain.
Key Players Profiled in the Cardiac Biomarkers Market
- Abbott Laboratories Inc.
- QuidelOrtho Corporation
- Siemens Healthineers AG
- F. Hoffmann-La Roche Ltd.
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- Randox Laboratories Ltd.
- Creative Diagnostics
- Life Diagnostics Inc.
- BIOMeRIEUX Inc.
Report Scope
In this report, the Global Cardiac Biomarkers Market has been segmented into the following categories:Cardiac Biomarkers Market, by Type:
- Troponin
- CK-MB
- Myoglobin
- BNP and NT-proBNP
- Others
Cardiac Biomarkers Market, by Application:
- Acute Coronary Syndrome
- Myocardial Infarction
- Congestive Heart Failure
- Others
Cardiac Biomarkers Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cardiac Biomarkers Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Cardiac Biomarkers market report include:- Abbott Laboratories Inc.
- QuidelOrtho Corporation
- Siemens Healthineers AG
- F. Hoffmann-La Roche Ltd
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- Randox Laboratories Ltd.
- Creative Diagnostics
- Life Diagnostics Inc.
- BIOMeRIEUX Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
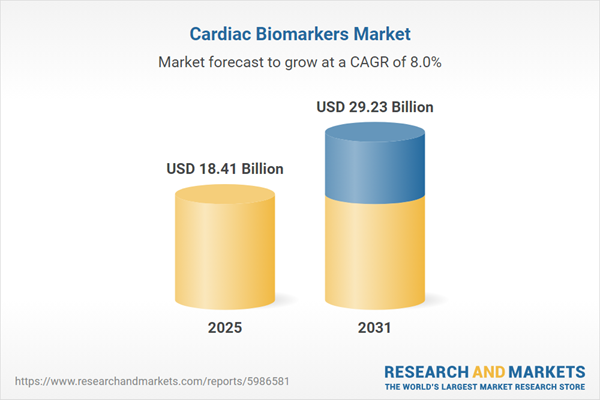
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 18.41 Billion |
| Forecasted Market Value ( USD | $ 29.23 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |