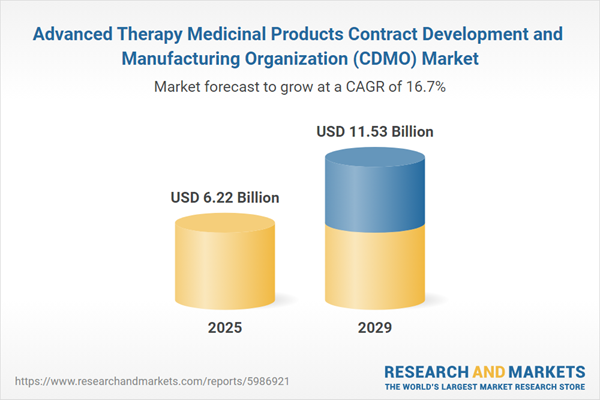
The advanced therapy medicinal products contract development and manufacturing organization (CDMO) market size has grown rapidly in recent years. It will grow from $5.31 billion in 2024 to $6.22 billion in 2025 at a compound annual growth rate (CAGR) of 17%. The growth in the historic period can be attributed to the increasing number of ATMP clinical trials, rising awareness and belief among researchers regarding the benefits of advanced therapy, the presence of several ATMP CDMOs, increasing efforts to develop novel therapies for various diseases, and supportive government initiatives.
The advanced therapy medicinal products contract development and manufacturing organization (CDMO) market size is expected to see rapid growth in the next few years. It will grow to $11.53 billion in 2029 at a compound annual growth rate (CAGR) of 16.7%. The growth in the forecast period can be attributed to the high investment of CDMOs, a growing number of emerging players in the cell and gene therapy (CGT) sector, increasing grants and investments for the development of cell and gene therapies, increasing pharmaceutical R&D spending, increasing number of clinical trials for ATMPs. Major trends in the forecast period include advancements in AAV biomanufacturing facilities, expansion of cell and gene therapy manufacturing, technological advancements in cell and gene therapy, increasing need for access to advanced platforms and technologies, and incorporating advanced technologies such as CRISPR and next-generation sequencing for heightened accuracy.
The growth of the advanced therapy medicinal products CDMO market is anticipated to be propelled by the increasing number of clinical trials. Clinical trials, which are research studies conducted in humans to assess the safety and efficacy of medical interventions such as drugs, treatments, devices, or preventive measures, are on the rise to meet the growing demand for innovative treatments and to tackle the increasing global disease burden. The utilization of advanced therapy medicinal products leads to a heightened demand for CDMO manufacturing services, which leverage specialized expertise and infrastructure. Clinical trial data assists in optimizing manufacturing processes, ensuring scalability, and maintaining quality to meet larger clinical and commercial demands. For example, in November 2023, the Association of the British Pharmaceutical Industry, a UK-based trade association, reported a marginal increase of 4.3% in the overall number of industry clinical trials initiated in the UK per year, rising from 394 in 2021 to 411 in 2022. Thus, the escalating number of clinical trials is propelling the growth of the advanced therapy medicinal products CDMO market.
Major players in the advanced therapy medicinal products CDMO market are focusing on developing advanced cell therapy manufacturing services to address critical issues in cell therapy manufacturing and accelerate the development activities of their partners. These cell therapy manufacturing services, offered by contract development and manufacturing organizations, encompass services ranging from early preclinical development to late-stage clinical trials and commercialization. For instance, in January 2024, Pluristem Therapeutics Inc., an Israel-based biotech company specializing in cell solutions, introduced PluriCDMO, a new business division offering cell therapy manufacturing services. PluriCDMO utilizes a patented bioreactor system enabling 3D cell multiplication, facilitating the production of various cell types such as stem cells, induced pluripotent stem cells, exosomes, and immune therapies. PluriCDMO aims to deliver high-quality medicines to patients while ensuring batch-to-batch consistency in a scalable and cost-effective manner within good manufacturing practice settings.
In February 2022, Recipharm AB, a Sweden-based contract development and manufacturing organization (CDMO), acquired Vibalogics for an undisclosed sum. This acquisition is intended to bolster Recipharm's presence in advanced therapy medicinal products and enable expansion into additional biologic modalities such as viral vectors. The acquisition will allow Recipharm to broaden its technologies and modalities, positioning it to better serve the growing demand for specialist CDMO capabilities in viral manufacturing within the rapidly expanding biopharmaceutical industry. Vibalogics is a Germany-based virotherapy CDMO specializing in manufacturing oncolytic viruses, viral vaccines, and gene therapies.
Major companies operating in the advanced therapy medicinal products contract development and manufacturing organization (CDMO) market are Thermo Fisher Scientific Inc., Merck KGaA, Resonac Corporation, Lonza Group AG, Catalent Inc., Charles River Laboratories International Inc., Recipharm AB, KBI Biopharma, Takara Bio Inc., Rentschler Biopharma SE, Ajinomoto Co. Inc., ElevateBio LLC, Fujifilm Diosynth Biotechnologies, Axplora Group GmbH, WuXi Advanced Therapies Inc., Celonic Group, Batavia Biosciences B.V., BioCentriq, Cytovance Biologics, Andelyn Biosciences, Genezen, AGC Biologics, ABL Manufacturing, Minaris Regenerative Medicine, Porton Advanced Solutions.
North America was the largest region in the advanced therapy medicinal product contract development and manufacturing organization (CDMO) market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the advanced therapy medicinal products contract development and manufacturing organization (CDMO) market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the advanced therapy medicinal products contract development and manufacturing organization (CDMO) market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
An advanced therapy medicinal product contract development and manufacturing organization (CDMO) is a specialized service provider that aids in the development and manufacturing of advanced therapy medicinal products. The objective of an advanced therapy medicinal product CDMO is to offer the expertise, infrastructure, and resources necessary for the development and production of these advanced therapies.
The main types of products handled by advanced therapy medicinal product CDMOs include gene therapy, cell therapy, and tissue-engineered products. Gene therapy involves the introduction, removal, or alteration of genetic material within a patient's cells to treat or prevent disease. This process can utilize viral or non-viral vectors to deliver therapeutic genes to target cells across various phases, such as phase I, phase II, phase III, and phase IV clinical trials. These therapies address a range of indications, including oncology, cardiology, central nervous system and musculoskeletal conditions, infectious diseases, dermatology, endocrine and metabolic disorders, genetic diseases, immunology and inflammation, ophthalmology, and hematology.
The advanced therapy medicinal products contract development and manufacturing organization (CDMO) market research report is one of a series of new reports that provides advanced therapy medicinal products contract development and manufacturing organization (CDMO) market statistics, including the advanced therapy medicinal products contract development and manufacturing organization (CDMO) industry global market size, regional shares, competitors with advanced therapy medicinal products contract development and manufacturing organization (CDMO) market share, detailed advanced therapy medicinal products contract development and manufacturing organization (CDMO) market segments, market trends, and opportunities, and any further data you may need to thrive in the advanced therapy medicinal products contract development and manufacturing organization (CDMO) industry. These advanced therapy medicinal products contract development and manufacturing organization (CDMO) market research reports deliver a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The advanced therapy medicinal product contract development and manufacturing organization (CDMO) market includes revenues earned by entities by providing services such as process development, analytical method development, good manufacturing practice manufacturing, and quality control testing. The market value includes the value of related goods sold by the service provider or included within the service offering. The advanced therapy medicinal products CDMO market also includes sales of bioreactors and fermenters, cryopreservation equipment, and laboratory instruments. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Advanced Therapy Medicinal Products Contract Development and Manufacturing Organization (CDMO) Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on advanced therapy medicinal products contract development and manufacturing organization (cdmo) market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for advanced therapy medicinal products contract development and manufacturing organization (cdmo) ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The advanced therapy medicinal products contract development and manufacturing organization (cdmo) market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Gene Therapy; Cell Therapy; Tissue Engineered2) By Phase: Phase I; Phase II; Phase III; Phase IV
3) By Indication: Oncology; Cardiology; Central Nervous System and Musculoskeletal; Infectious Disease; Dermatology; Endocrine, Metabolic, Genetic; Immunology and Inflammation; Ophthalmology; Hematology
Subsegments:
1) By Gene Therapy: Viral Vectors; Non-Viral Vectors; Dna or Rna-Based Products2) By Cell Therapy: Autologous Cell Therapy; Allogeneic Cell Therapy; Stem Cell Therapy
3) By Tissue Engineered: Skin Substitutes; Cartilage and Bone Tissue; Vascular Grafts and Organs
Key Companies Mentioned: Thermo Fisher Scientific Inc.; Merck KGaA; Resonac Corporation; Lonza Group AG; Catalent Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Advanced Therapy Medicinal Products Contract Development and Manufacturing Organization (CDMO) market report include:- Thermo Fisher Scientific Inc.
- Merck KGaA
- Resonac Corporation
- Lonza Group AG
- Catalent Inc.
- Charles River Laboratories International Inc.
- Recipharm AB
- KBI Biopharma
- Takara Bio Inc.
- Rentschler Biopharma SE
- Ajinomoto Co. Inc.
- ElevateBio LLC
- Fujifilm Diosynth Biotechnologies
- Axplora Group GmbH
- WuXi Advanced Therapies Inc.
- Celonic Group
- Batavia Biosciences B.V.
- BioCentriq
- Cytovance Biologics
- Andelyn Biosciences
- Genezen
- AGC Biologics
- ABL Manufacturing
- Minaris Regenerative Medicine
- Porton Advanced Solutions
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 6.22 Billion |
| Forecasted Market Value ( USD | $ 11.53 Billion |
| Compound Annual Growth Rate | 16.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |