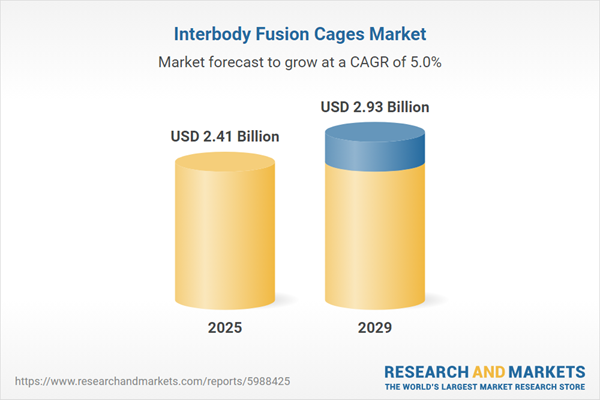
The interbody fusion cages market size has grown strongly in recent years. It will grow from $2.29 billion in 2024 to $2.41 billion in 2025 at a compound annual growth rate (CAGR) of 5.2%. The growth in the historic period can be attributed to an increase in the geriatric population, an increase in obesity rates, a surge in sports-related injuries, economic growth, and hospital infrastructure development.
The interbody fusion cages market size is expected to see strong growth in the next few years. It will grow to $2.93 billion in 2029 at a compound annual growth rate (CAGR) of 5%. The growth in the forecast period can be attributed to growing awareness of minimally invasive surgical procedures, increasing number of spinal surgeries, rising number of accidents, upsurge in disposable income, and increasing prevalence of degenerative diseases. Major trends in the forecast period include technological advancements, integrated graft delivery systems, strategic collaborations, minimally invasive approaches, and modular and adjustable designs.
The increase in spinal cord injuries is anticipated to drive the expansion of the interbody fusion cages market in the future. Spinal cord injuries result from damage to the spinal cord, a complex network of nerves transmitting signals from the brain to the body's organs. The escalation in spinal cord injuries is attributed to falls, traffic accidents, sports and recreational activities, workplace accidents, violence, illnesses, and medical conditions. Interbody fusion cages play a crucial role in treating spinal cord injuries by promoting bone fusion, offering structural support, and enhancing the stability and functionality of the spine. For example, in May 2023, as reported by Spinal Cord Inc., a US-based medical information provider, there were approximately 18,000 new spinal cord injuries annually in the United States, affecting a population of around 330 million residents each year. Thus, the increase in spinal cord injuries is expected to drive the growth of the interbody fusion cages market.
Leading companies in the interbody fusion cages market are innovating by introducing advanced products, such as expanding interbody cages, to better meet the needs of their customers. An expanding interbody cage, also referred to as an expandable interbody cage, is a type of spinal implant utilized in fusion procedures that can increase in size after being placed into the disc space between two vertebrae. For example, in May 2022, Accelus, a US-based manufacturer of medical equipment, unveiled the TiHawk7 Titanium, a bonded multidirectional expanding interbody cage designed to facilitate endoscopic and minimally invasive spine (MIS) lumbar fusion procedures. This groundbreaking device features an ultra-low insertion profile with multidirectional interbody expansion, utilizing both titanium and Polyether ether ketone (PEEK) materials to ensure optimal performance. The device's distinctive attributes include an ultra-low insertion profile, an open-architecture design to maximize graft delivery, and an innovative titanium application.
In October 2023, Silony Medical International AG, a medical device company headquartered in Switzerland, purchased the fusion business and associated assets from Centinel Spine for an undisclosed sum. Through this acquisition, Silony intends to broaden its range of offerings in the spine surgery sector and reinforce its position as a leading supplier of cutting-edge medical devices for spinal fusion operations. Centinel Spine, based in the US, is a medical device company specializing in an interbody fusion platform.
Major companies operating in the interbody fusion cages market are Medtronic plc, Johnson & Johnson, Stryker Corporation, B. Braun Melsungen AG, Depuy Synthes, Shanghai MicroPort Orthopedics Co. Ltd., Zimmer Biomet Holdings Inc., NuVasive Inc., Medacta International SA, Alphatec Spine Inc., SeaSpine Orthopedics Corporation, Orthofix International N.V., Surgalign Spine Technologies, Rivarp Medical Private Limited, Baumer S.A., Medicrea International, Precision Spine Inc., Spineart SA, Benvenue Medical Inc., Aurora Spine Inc., Ulrich Medical USA, Elite surgical, CarboFix Orthopedics Ltd., Prodorth International Inc., K2M Group Holdings Inc., Biomet Inc., Intelivation LLC, Eminent Spine.
North America was the largest region in the interbody fusion cages market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the interbody fusion cages market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the interbody fusion cages market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Interbody fusion cages are implants utilized in spinal fusion surgery to encourage the fusion of neighboring vertebral bones within the spine. Crafted from biocompatible materials such as titanium or polymer, these devices are placed into the intervertebral space between two vertebrae to offer structural support and encourage bone growth. The primary objectives of interbody fusion cages are to stabilize the spine, alleviate discomfort, and reinstate spinal alignment and functionality.
The primary categories within the interbody fusion cages market comprise lumbar cages, cervical cages, thoracolumbar cages, and thoracic cages. Lumbar cages are tailored interbody fusion devices intended for the lumbar portion of the spine, offering support and stability to the lower back region. These devices are utilized in various surgical approaches including anterior, posterior, lateral, and transforaminal procedures, serving end-users such as hospitals, clinics, ambulatory surgical centers, and other facilities.
The interbody fusion cages market research report is one of a series of new reports that provides interbody fusion cages market statistics, including interbody fusion cages industry global market size, regional shares, competitors with an interbody fusion cages market share, detailed interbody fusion cages market segments, market trends, and opportunities, and any further data you may need to thrive in the interbody fusion cages industry. This interbody fusion cage research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The interbody fusion cages market consists of sales of pedicle screws and rods, interbody cages, and spinal fusion plates. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Interbody Fusion Cages Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on interbody fusion cages market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for interbody fusion cages ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The interbody fusion cages market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Lumbar Cage; Cervical Cage; Thoraco-Lumbar Cage; Thoracic Cage2) By Surgery: Anterior; Posterior; Lateral; Transforaminal
3) By End Users: Hospitals and Clinics; Ambulatory Surgical Center; Other End-Users
Subsegments:
1) By Lumbar Cage: Posterior Lumbar Interbody Fusion (PLIF) Cage; Transforaminal Lumbar Interbody Fusion (TLIF) Cage; Anterior Lumbar Interbody Fusion (ALIF) Cage; Oblique Lumbar Interbody Fusion (OLIF) Cage2) By Cervical Cage: Anterior Cervical Interbody Fusion (ACIF) Cage; Posterior Cervical Interbody Fusion (PCIF) Cage
3) By Thoraco-Lumbar Cage: Expandable Thoraco-Lumbar Cage; Static Thoraco-Lumbar Cage
4) By Thoracic Cage: Anterior Thoracic Interbody Fusion Cage; Posterior Thoracic Interbody Fusion Cage
Key Companies Mentioned: Medtronic plc; Johnson & Johnson; Stryker Corporation; B. Braun Melsungen AG; Depuy Synthes
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Interbody Fusion Cages market report include:- Medtronic plc
- Johnson & Johnson
- Stryker Corporation
- B. Braun Melsungen AG
- Depuy Synthes
- Shanghai MicroPort Orthopedics Co. Ltd.
- Zimmer Biomet Holdings Inc.
- NuVasive Inc.
- Medacta International SA
- Alphatec Spine Inc.
- SeaSpine Orthopedics Corporation
- Orthofix International N.V.
- Surgalign Spine Technologies
- Rivarp Medical Private Limited
- Baumer S.A.
- Medicrea International
- Precision Spine Inc.
- Spineart SA
- Benvenue Medical Inc.
- Aurora Spine Inc.
- Ulrich Medical USA
- Elite surgical
- CarboFix Orthopedics Ltd.
- Prodorth International Inc.
- K2M Group Holdings Inc.
- Biomet Inc.
- Intelivation LLC
- Eminent Spine
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.41 Billion |
| Forecasted Market Value ( USD | $ 2.93 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |