Clot Management Devices Market Overview
Clot management devices are used for the treatment of blood clots to prevent conditions like stroke, deep vein thrombosis, and pulmonary embolism. It offers rapid blood flow restoration and minimal invasive options. Improvements in imaging as well as device flexibility help in improving the efficacy of treatment.Blood clots are semi-solid clumps of blood. Blood normally flows via arteries and veins without restriction. A certain amount of clotting is required to halt the bleeding if you get hurt or cut. But excessive clotting can result in serious problems. The blood clot forms can be stationary which is known as thrombosis, or it can obstruct blood flow, or it can become loose (known as embolism) and travel to various parts of the body. Clots can obstruct the flow of blood, causing major health concerns like heart attacks and strokes. Clot management devices play a crucial in avoiding such conditions by removing or dissolving clots effectively.
Clot Management Devices Market Growth Drivers
Increasing Incidence of Cardiovascular Diseases (CVDs) Affects the Market Landscape Significantly
The market is driven by the high prevalence of CVDs, technological advancements as well as increasing demand from the aging population and stroke. According to the new Global Burden of Disease (GBD) special report published in December 2023, cardiovascular disease remains a leading cause of death. There is an estimate of 17 million deaths a year globally due to CVDs out of which 25% are sudden cardiac deaths (SCD). Heart attack and stroke account for 85% of cardiovascular deaths.The rising burden of CVDs significantly influences device adoption and innovation. Due to the rising prevalence of CVDs, more people are susceptible to blood clots which increases the need for clot management devices in both treatment and prevention.
Ease in Government Regulation to meet Rising Global Clot Management Devices Market Demand
Government initiatives and reimbursement policies are driving market growth. Public health campaigns increase demand for early clot detection and treatment, while government funding and grants facilitate innovation. Improved healthcare infrastructure, particularly in developing regions, improves access to quality medical treatments. Favorable reimbursement policies cover advanced procedures, making them more affordable. Financial incentives for healthcare providers and patient assistance programs increase accessibility and adherence to prescribed therapies. These initiatives enhance healthcare standards globally.Clot Management Devices Market Trends
The market is witnessing several trends and developments to improve the current global scenario. Some of the notable trends are as follows:Technological Advancements
Technical advancements have enhanced patient care by facilitating safer removal, accurate identification, and fewer side effects. Artificial intelligence and bioresorbable stents are among new developments. In addition, tools for diagnosis and treatment planning are also using machine learning.Rise in Minimally Invasive Procedures
Minimally invasive procedures are becoming more popular than traditional open surgeries, with procedures such as catheter-directed thrombolysis (CDT) and pharmacomechanical thrombolysis (PMT) garnering popularity.Expanding Applications
These devices are being used in various medical conditions, including cancer-related thrombosis as well as peripheral artery disease . Research and clinical trials are expanding their efficiency, exploring novel applications in oncology and peripheral vascular interventions which enhance patient care and encouraging innovation in medical device technology.Growing Awareness and Screening Programs
An aging world population makes clot-related conditions like venous thromboembolism and atrial fibrillation more common. As people become aware of the risks of blood clots and importance of early diagnosis and treatment, there are more screenings and interventions.Clot Management Devices Market Segmentation
The report titled “Clot Management Devices Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Neurovascular Embolectomy Devices
- Embolectomy Balloon Catheters
- Percutaneous Thrombectomy Devices
- Catheter-Directed Thrombolysis (CDT) Devices
- Inferior Vena Cava Filters (IVCF)
Market Breakup by End-user
- Hospitals
- Specialty Clinics
- Ambulary Surgical Centers
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Clot Management Devices Market Share
Hospitals Segment Based on End-Users Holds a Significant Market Share
End users in the global clot management device market include hospitals, specialized clinics, ambulatory surgery centers (ASCs), and others. Hospitals are poised to hold a significant market share because of their extensive facilities and ability to manage complex operations, ensuring a high rate of use.Specialty clinics are also poised to hold a notable market share as they offer focused care using specialized equipment and experience. These clinics are specialized in the diagnosis and treatment of conditions associated with blood clotting, deep vein thrombosis (DVT), venous thromboembolism (VTE), and other vascular problems.
Ambulatory surgery centers also use clot management devices and play a crucial role in the market because they make minimally invasive operations like thrombectomy and angioplasty affordable. With the use of cutting-edge medical technology, they can offer same-day surgery with shorter recovery periods and a lower risk of infection, improving patient outcomes and increasing the effectiveness and accessibility of clot management therapies.
Other end users, particularly diagnostic centers, make substantial contributions by providing critical diagnostic and minor treatment choices, thereby assisting the significant healthcare ecosystem in treating clot-related illnesses.
Clot Management Devices Market Analysis by Region
Based on region, the market report covers North America, Europe, Asia Pacific, Latin America along with the Middle East and Africa.North America holds the largest market share due to its high healthcare spending, an aging population, and a high incidence of cardiovascular disorders such as heart attacks and strokes. The region benefits from considerable technological breakthroughs and substantial investment from major industry participants.
Europe also have a significant market share with major players including Germany, UK, France, and Italy. Rising cardiovascular disease rates, a well-established healthcare system, and a high demand for minimally invasive procedures are driving the region's market rise.
The Asia Pacific region is experiencing the fastest growth in market due to surge in healthcare investments, improved infrastructure, and rising heart disease rates in countries like China, Japan, and India. This trend is also driven by increased patient numbers in emerging economies.
Leading Players in the Global Clot Management Devices Market
The key features of the market report include patent analysis, as well as strategic initiatives including recent partnerships and collaborations by the leading players. The major companies in the market are as follows:iVascular
iVascular is a fast-growing company established in 2010 in Barcelona, Spain. It is well-known for its innovations in vascular devices, which include clot management systems such as mechanical thrombectomy devices, thrombolysis systems, and thrombectomy catheters, which are used in cardiovascular and vascular diseases. These devices help to dissolve and remove clots, which improves patient outcomes for diseases like deep vein thrombosis, heart attacks, and strokes.Boston Scientific Corporation
It is a is a biomedical/biotechnology engineering firm which was established in Delaware in 1979 and has its headquarters in Watertown, Massachusetts. It is known for its large range of medical equipment. It is a key player in the clot management market, particularly in thrombectomy and embolization devices.Medtronic
Medtronic was established in 1949. It is a key player in the medical device sector, offering a range of clot management devices and actively involved in product development and market expansion.AngioDynamics
AngioDynamics was founded in 1988 and is headquartered in Latham, New York, USA. It is a medical technology company that focuses on developing, manufacturing, and marketing minimally invasive medical devices for vascular diseases and oncology. They offer clot management devices, including thrombolytic catheters, thrombectomy devices, and venous access devices, used by healthcare professionals to prevent, diagnose, and treat blood clots in various body parts, including veins and arteries.Other players in the market include BD, Cardinal Health, Cook Medical, Edwards Lifesciences Corporation, Johnson & Johnson Services Inc, LeMaitre Vascular Inc, Stryker, Teleflex Incorporated.
Key Questions Answered in the Global Clot Management Devices Market Report
- What was the global clot management devices market value in 2024?
- What is the global clot management devices market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on products?
- Who are the major end users in the market?
- What are the major factors aiding the global clot management devices market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major trends influencing the market?
- What are the major drivers, opportunities, and restraints in the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Which end user will contribute significantly to the market growth?
- Who are the key players involved in the clot management devices market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- iVascular.
- Boston Scientific Corporation
- Medtronic
- Teleflex Incorporated.
- Edwards Lifesciences Corporation.
- LeMaitre Vascular, Inc
- BD
- Cook Medical
- Johnson & Johnson Services, Inc.
- Stryker
- Cardinal Health.
- AngioDynamics.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
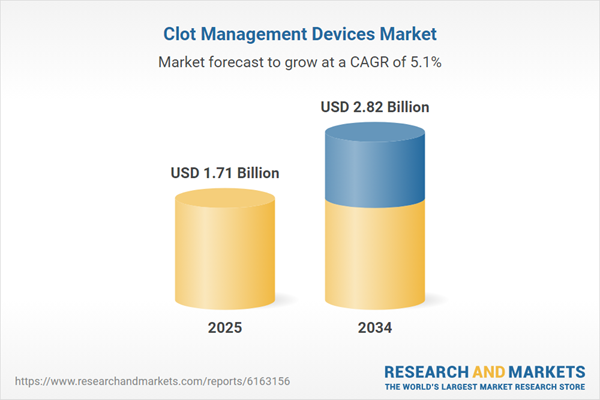
| Estimated Market Value ( USD | $ 1.71 Billion |
| Forecasted Market Value ( USD | $ 2.82 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |