Europe Generic Drugs Market Analysis
Generic drugs have the same active ingredients as present in the brand-name drugs. They are usually marketed at a lower cost, making them a cost-effective alternative to achieve substantial reductions in medical expenses. Generic pharmaceutical manufacturers must prove the bioequivalence of their version of a drug to a health regulatory agency such as the European Medicines Agency (EMA) for marketing authorization. The increasing government initiatives and the ongoing efforts to establish regulatory reforms that promote the use of generic medications are significantly contributing to the Europe generic drugs market growth.In Europe, the rising healthcare needs which can be attributed to the growing prevalence of chronic diseases and increasing aging population size are stimulating the global generic drugs market expansion. Recent data reveals that over 9 million adults will be affected by a major illness in England by 2040, accounting for 1 in 5 adults suffering from a health issue. Further, it is estimated that ill health will increase by 2.5 million in the adult population over the two decades from 2019, including a rise in the prevalence of cancer, diabetes, or kidney disease, by more than 30%. Thus, with the growing burden of chronic conditions, the need for affordable generic drugs is expected to fuel in the coming years. Additionally, th growth in the elderly population, as evidenced by one-fifth (21.3%) of the European Union population estimated to be aged 65 years and over in January 2023, is anticipated to augment the Europe generic drugs market demand in the forecast period.
One of the major market trends is the rise in patent expiration of branded drugs that allow generic drug manufacturers to produce and market a bioequivalent version of the same drug at a cheaper price. In 2024, Europe is reported to lose the market exclusivity of a wide range of drugs. Around 73 such drug patents across diverse therapeutic categories including established medications like Cabometyx (anti-cancer medication) and Zavicefta (antibiotic medication for severe bacterial infections) are set to expire, which is poised to significantly influence the market dynamics by facilitating market entry of generic drugs. However, the European market is facing increased generic product recalls, which can be tackled by the introduction of an optimized regulatory framework that ensures sustained growth of the market for generic drugs.
Europe Generic Drugs Market Segmentation
The report offers a detailed analysis of the market based on the following segments:Market Breakup by Therapy Area
- Cardiovascular
- Dermatology
- Respiratory
- Oncology
- Rheumatology
- Others
Market Breakup by Route of Administration
- Oral
- Injectables
- Dermal/Topical
- Inhalers
- Others
Market Breakup by Distribution Channels
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Market Breakup by Region
- United Kingdom
- Germany
- France
- Others
Leading Players in the Europe Generic Drugs Market
The key features of the market report include patent analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Teva Pharmaceutical Industries Ltd
- Viatris Inc.
- Sun Pharmaceutical Industries Ltd
- Lupin
- AstraZeneca
- Baxter
- Takeda Pharmaceutical Company Limited
- GSK plc
- Bausch + Lomb
- Novartis AG
- Sanofi
- Pfizer Inc.
- Fresenius SE & Co. KGaA
- Hikma Pharmaceuticals PLC
- Aurobindo Pharma
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Teva Pharmaceutical Industries Ltd
- Viatris Inc.
- Sun Pharmaceutical Industries Ltd
- Lupin
- AstraZeneca
- Baxter
- Takeda Pharmaceutical Company Limited
- GSK plc
- Bausch + Lomb
- Novartis AG
- Sanofi
- Pfizer Inc.
- Fresenius SE & Co. KGaA
- Hikma Pharmaceuticals PLC
- Aurobindo Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
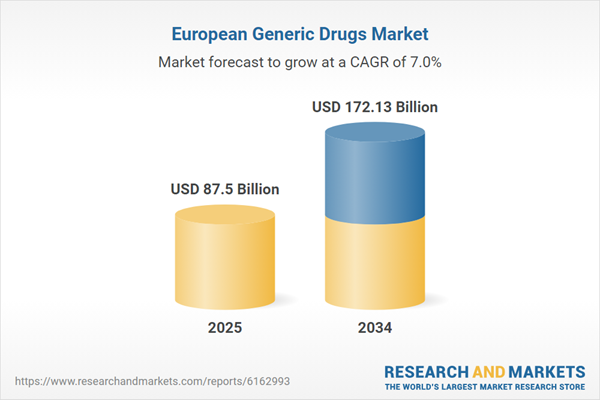
| Estimated Market Value ( USD | $ 87.5 Billion |
| Forecasted Market Value ( USD | $ 172.13 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 15 |