Global Trauma and Extremities Devices Market Analysis
The global trauma and extremities devices market encompasses a wide range of medical devices used to treat injuries and conditions affecting the bones, joints, and soft tissues of the extremities (arms, legs, hands, and feet). These devices include internal and external fixation devices, plates, screws, rods, and prosthetics. The market is driven by the increasing incidence of trauma cases, advancements in medical technology, and a growing aging population.Market Drivers
- Rising Incidence of Trauma Cases: The increasing number of road accidents, sports injuries, and falls, particularly among the elderly, drives the demand for trauma and extremities devices. These incidents often result in fractures and injuries requiring surgical intervention and specialized devices for treatment.
- Aging Population: The global aging population is more susceptible to fractures and orthopedic conditions due to decreased bone density and muscle strength. This demographic trend significantly boosts the demand for trauma and extremities devices.
- Technological Advancements: Innovations in medical technology, such as the development of bioabsorbable implants, 3D-printed devices, and advanced imaging techniques, enhance the effectiveness and precision of trauma and extremities treatments. These advancements improve patient outcomes and drive market growth.
- Increasing Healthcare Expenditure: Rising healthcare expenditure globally, particularly in emerging markets, supports the growth of the trauma and extremities devices market. Improved healthcare infrastructure and access to advanced medical treatments contribute to market expansion.
- Growing Awareness and Early Diagnosis: Increased awareness about the benefits of early diagnosis and treatment of orthopedic conditions drives the demand for trauma and extremities devices. Public health initiatives and educational campaigns contribute to this trend.
Market Challenges
- High Cost of Devices and Procedures: The high cost of trauma and extremities devices, coupled with expensive surgical procedures, can be a barrier to market growth, especially in low-income regions. Affordability issues limit access to advanced treatments for many patients.
- Stringent Regulatory Requirements: The stringent regulatory landscape for medical devices, including the approval process and compliance with safety standards, can delay product launches and increase development costs. Navigating these regulations poses a significant challenge for manufacturers.
- Risk of Complications and Revisions: The risk of post-surgical complications, such as infections and device failures, can affect patient outcomes and lead to additional surgeries. These risks may deter patients and healthcare providers from opting for surgical interventions.
- Limited Awareness in Developing Regions: In many developing regions, awareness about advanced trauma and extremities treatments is limited. Lack of education and inadequate healthcare infrastructure hinder market growth in these areas.
Future Opportunities
- Emerging Markets Expansion: Emerging markets in Asia-Pacific, Latin America, and Africa present significant growth opportunities due to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about advanced treatments. Manufacturers are focusing on expanding their presence in these regions to tap into the growing demand.
- Development of Cost-Effective Solutions: Developing cost-effective trauma and extremities devices can increase accessibility and affordability, particularly in low-income regions. Innovations aimed at reducing manufacturing costs and optimizing treatment protocols will drive market growth.
- Personalized and Patient-Specific Devices: The trend towards personalized medicine and customized treatment solutions is creating opportunities for the development of patient-specific trauma and extremities devices. Advances in 3D printing and biomaterials enable the production of customized implants that improve fit and functionality.
- Minimally Invasive Surgical Techniques: The adoption of minimally invasive surgical techniques for trauma and extremities treatments is growing. These techniques offer benefits such as reduced recovery times, less postoperative pain, and lower complication rates. Training programs and certifications for surgeons in these techniques will support this trend.
- Integration of Digital Health Technologies: The integration of digital health technologies, such as telemedicine, remote monitoring, and advanced imaging, can enhance the diagnosis, treatment, and postoperative care of trauma patients. These technologies improve patient outcomes and drive the adoption of advanced trauma and extremities devices.
Global Trauma and Extremities Devices Market Trends
- Adoption of Minimally Invasive Techniques
- Advancements in Bioabsorbable Implants
- Integration of 3D Printing Technology
- Increasing Use of Robotics and Computer-Assisted Surgery
- Growth of Outpatient and Ambulatory Surgery Centers
- Expansion of Telemedicine and Remote Monitoring
- Emphasis on Patient-Specific and Personalized Care
Global Trauma and Extremities Devices Market Segmentation
Market Breakup by Device Type
- External Fixation
- Internal Fixation
- Craniofacial Devices
- Long Bone Stimulation
- Others
Market Breakup by Surgical Site
- Upper Extremities
- Lower Extremities
- Others
Market Breakup by End User
- Hospitals
- Clinics
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Trauma and Extremities Devices Market Competitive Landscape
The trauma and extremities devices market features key players such as Stryker, Johnson & Johnson Services, Inc., Smith+Nephew, Tecomet, Inc., Citieffe s.r.l, CONMED Corporation, JEIL MEDICAL CORPORATION, Osteomed, Medartis AG, Colson Medical, Inc., Bioretec Ltd, Electramed Ltd, Zimmer Biomet, Tyber Medical LLC, and Extremity Medical. Common market activities among these companies include mergers and acquisitions to expand market presence and capabilities, extensive research initiatives to innovate and improve device offerings, new product introductions to address evolving clinical needs, and strategic partnerships with healthcare providers and research institutions to enhance market reach and drive technological advancements. These strategies collectively enhance competitive positioning and drive market growth.Key Questions Answered in the Report
- What is the current and future performance of the trauma and extremities devices market?
- What are the main challenges facing the trauma and extremities devices market?
- What are the key drivers of the trauma and extremities devices market?
- What emerging trends are shaping the future of the trauma and extremities devices market?
- How are minimally invasive techniques improving trauma and extremities treatments and patient recovery times?
- Why are bioabsorbable implants becoming popular in the trauma and extremities devices market?
- How are robotic and computer-assisted systems improving precision in trauma and extremities surgeries?
- Why are craniofacial devices and long bone stimulation devices becoming more popular in trauma treatment?
- Why are clinics becoming more popular for specialized and outpatient care for minor fractures and injuries?
- What are the common strategies used by key players in the trauma and extremities devices market?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the global trauma and extremities devices market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the trauma and extremities devices market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the trauma and extremities devices industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Stryker Corp (NYSE: SYK)
- Zimmer Biomet Holding Inc. (NYSE: ZBH)
- Smith & Nephew PLC (LON: SN)
- Wright Medical Group N.V. (NASDAQ: WMGI)
- Advanced Orthopaedic Solutions
- Johnson & Johnson Medtech (DePuy Synthes)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | July 2024 |
| Forecast Period | 2024 - 2032 |
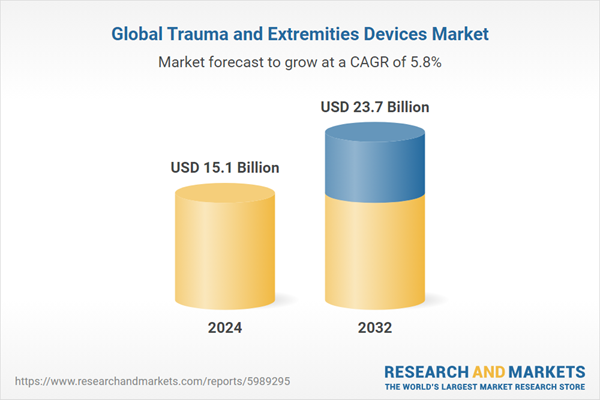
| Estimated Market Value ( USD | $ 15.1 Billion |
| Forecasted Market Value ( USD | $ 23.7 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 6 |