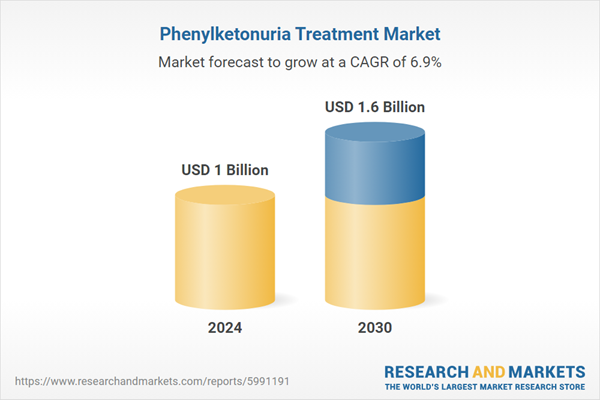
Global Phenylketonuria Treatment Market - Key Trends and Drivers Summarized
Phenylketonuria (PKU) is a rare inherited metabolic disorder caused by a deficiency in the enzyme phenylalanine hydroxylase (PAH), which is necessary for the metabolism of the amino acid phenylalanine. When PAH is deficient or absent, phenylalanine accumulates in the blood and brain, leading to severe cognitive impairments and neurological issues if left untreated. The cornerstone of PKU management has traditionally been a strict, lifelong diet low in phenylalanine, which involves the exclusion of high-protein foods and the inclusion of specially formulated medical foods and phenylalanine-free amino acid supplements. Early diagnosis through newborn screening programs has been critical in preventing the severe outcomes associated with untreated PKU, allowing for the timely initiation of dietary management and close monitoring of phenylalanine levels.In recent years, advances in PKU treatment have expanded beyond dietary management, providing new therapeutic options that improve patient outcomes and quality of life. One significant development is the use of pharmacological treatments such as sapropterin dihydrochloride, a synthetic form of the cofactor tetrahydrobiopterin (BH4), which can enhance the residual activity of PAH in some patients with mild to moderate PKU. Additionally, pegvaliase, an enzyme substitution therapy, has been approved for adults with PKU who have uncontrolled blood phenylalanine levels despite dietary management. Pegvaliase works by breaking down phenylalanine in the bloodstream, offering a new avenue for those who struggle to maintain low phenylalanine levels through diet alone. Gene therapy and other innovative treatments are also under investigation, aiming to provide more permanent solutions by addressing the underlying genetic cause of PKU.
The growth in the phenylketonuria treatment market is driven by several factors. The increasing awareness and understanding of PKU among healthcare providers and patients have led to earlier and more accurate diagnoses, boosting the demand for effective treatments. Technological advancements and ongoing research have facilitated the development of new pharmacological therapies and innovative treatments, expanding the options available for PKU management. The rise of personalized medicine, which tailors treatments to individual genetic profiles, is also driving market growth by offering more targeted and effective therapies. Additionally, the expansion of newborn screening programs globally has increased the detection rates of PKU, further fueling the need for comprehensive treatment solutions. The growing availability of specialized medical foods and supplements, coupled with supportive healthcare policies and funding for rare disease treatments, are creating a favorable market environment. As these trends continue to evolve, the phenylketonuria treatment market is poised for significant growth, driven by advancements in medical science and a heightened focus on improving patient outcomes.
Report Scope
The report analyzes the Phenylketonuria Treatment market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Drug Type (Palynziq, Kuvan, Other Drug Types).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Palynziq segment, which is expected to reach US$918.2 Million by 2030 with a CAGR of a 7.3%. The Kuvan segment is also set to grow at 6.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $282.5 Million in 2024, and China, forecasted to grow at an impressive 11% CAGR to reach $343.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Phenylketonuria Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Phenylketonuria Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Phenylketonuria Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ajinomoto Cambrooke, Inc., American Gene Technologies Inc., Arla Foods Ingredients Group P/S, BioMarin Pharmaceutical, Inc., Codexis, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Phenylketonuria Treatment market report include:
- Ajinomoto Cambrooke, Inc.
- American Gene Technologies Inc.
- Arla Foods Ingredients Group P/S
- BioMarin Pharmaceutical, Inc.
- Codexis, Inc.
- Metropolis Healthcare Ltd.
- Nestle Health Science SA
- Nutricia
- PTC Therapeutics
- SOM Biotech
- Synlogic
- Travere Therapeutics, Inc.
- Ultragenyx Pharmaceutical, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ajinomoto Cambrooke, Inc.
- American Gene Technologies Inc.
- Arla Foods Ingredients Group P/S
- BioMarin Pharmaceutical, Inc.
- Codexis, Inc.
- Metropolis Healthcare Ltd.
- Nestle Health Science SA
- Nutricia
- PTC Therapeutics
- SOM Biotech
- Synlogic
- Travere Therapeutics, Inc.
- Ultragenyx Pharmaceutical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1 Billion |
| Forecasted Market Value ( USD | $ 1.6 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |