Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Male urinary incontinence, characterized by the involuntary leakage of urine, is a common condition, primarily affecting aging men. This demographic shift has resulted in a substantial increase in the prevalence of this condition, driving the demand for effective treatment options like male artificial urinary sphincters.
Key Market Drivers
Aging Population
The aging population is a significant driver of growth in the North America Male Artificial Urinary Sphincter Market. As individuals age, they are more prone to various health conditions, including urinary incontinence, which often necessitates medical intervention such as the implantation of artificial urinary sphincters. This demographic trend creates a growing market for these devices and associated procedures.One key factor contributing to the increased prevalence of urinary incontinence among the aging population is the natural aging process itself. As people age, the muscles and nerves that control bladder function may weaken, leading to involuntary urine leakage. Age-related medical conditions such as prostate enlargement in men and pelvic floor disorders in both men and women can further exacerbate urinary incontinence. The aging population is also characterized by a higher incidence of medical conditions and comorbidities that can contribute to urinary incontinence. Chronic conditions such as diabetes, neurological disorders, and cardiovascular diseases become more prevalent with age, and these conditions can directly or indirectly affect bladder function, increasing the likelihood of urinary incontinence. Older adults often face challenges such as mobility limitations, cognitive impairments, and social isolation, which can exacerbate urinary incontinence and decrease their quality of life. As a result, there is a growing demand for effective treatments that can help manage urinary incontinence and improve overall well-being among the aging population.
Artificial urinary sphincters are among the treatment options available for managing urinary incontinence, particularly in cases where conservative measures such as lifestyle modifications and medications have been ineffective. These devices mimic the function of the natural urinary sphincter by regulating the flow of urine from the bladder, thereby helping to control urinary leakage. The aging population's increasing awareness and acceptance of medical interventions for urinary incontinence also contribute to market growth. As older adults become more informed about available treatment options and seek to maintain their independence and quality of life, they are more likely to consider surgical interventions such as artificial urinary sphincter implantation.
Advancements in medical technology have led to the development of more sophisticated and minimally invasive artificial urinary sphincters, which appeal to older adults seeking effective and less invasive treatment options. These technological innovations not only improve patient outcomes but also enhance the overall patient experience, leading to greater patient satisfaction and increased demand for these devices. The rising healthcare expenditure associated with the aging population further drives market growth. As healthcare systems strive to meet the growing demand for medical services among older adults, there is an increased focus on improving access to specialized treatments such as artificial urinary sphincter implantation. This investment in healthcare infrastructure and services creates a conducive environment for market expansion.
Also, demographic shifts such as the increasing proportion of older adults in the population pyramid contribute to market growth by creating a larger target market for artificial urinary sphincter manufacturers and healthcare providers. This demographic trend is expected to continue in the coming years, further fueling demand for these devices and procedures. The aging population is a significant driver of growth in the North America Male Artificial Urinary Sphincter Market. As older adults face an increased risk of urinary incontinence due to age-related changes and medical conditions, there is a growing demand for effective treatments such as artificial urinary sphincters. Demographic shifts, technological advancements, and healthcare expenditure patterns further contribute to market expansion. As the aging population continues to grow, the market for artificial urinary sphincters is expected to expand, offering opportunities for manufacturers, healthcare providers, and other stakeholders in the healthcare industry.
Advancements in Healthcare Technology
Advancements in healthcare technology play a pivotal role in driving the growth of the North America Male Artificial Urinary Sphincter Market. These advancements encompass a wide range of innovations, including improvements in the design and functionality of artificial urinary sphincters, advancements in surgical techniques, and the integration of digital health solutions. Together, these technological advancements enhance the efficacy, safety, and accessibility of artificial urinary sphincter procedures, thereby expanding the market and improving patient outcomes.One key area of advancement in healthcare technology is the development of more sophisticated and minimally invasive artificial urinary sphincters. Traditional artificial urinary sphincters consisted of mechanical devices that required manual manipulation to regulate urinary flow. However, recent technological innovations have led to the introduction of programmable and adjustable devices that offer greater control and customization for patients. These next-generation artificial urinary sphincters utilize advanced materials and electronics to provide precise and reliable urinary continence control while minimizing the risk of complications. Advancements in surgical techniques, such as the adoption of laparoscopic and robotic-assisted procedures, have contributed to the growth of the Male artificial urinary sphincter market. These minimally invasive approaches allow for smaller incisions, reduced postoperative pain, and faster recovery times compared to traditional open surgeries. As a result, more patients are able to undergo artificial urinary sphincter implantation with improved outcomes and a lower risk of complications.
In addition to improvements in device design and surgical techniques, the integration of digital health solutions has also played a significant role in driving market growth. Digital health technologies, such as telemedicine platforms, remote monitoring devices, and mobile health apps, enable healthcare providers to deliver more personalized and accessible care to patients undergoing artificial urinary sphincter procedures. For example, telemedicine platforms allow patients to consult with healthcare providers remotely, reducing the need for in-person visits and improving access to specialist care, particularly in rural or underserved areas. Remote monitoring devices enable healthcare providers to track patients' progress and adjust treatment plans accordingly, enhancing postoperative management and reducing the risk of complications.
Mobile health apps provide patients with valuable resources and support tools to manage their condition effectively. These apps may include features such as symptom tracking, medication reminders, and educational content, empowering patients to take an active role in their healthcare journey and improve treatment adherence. The integration of electronic health records (EHRs) and health information exchange (HIE) systems also facilitates seamless communication and collaboration among healthcare providers involved in the care of patients undergoing artificial urinary sphincter procedures. This interoperability enables the sharing of patient data, treatment plans, and outcomes across different healthcare settings, ensuring continuity of care and optimizing clinical decision-making.
Also, advancements in healthcare technology have led to improvements in patient education and engagement, which are essential for driving adoption of artificial urinary sphincter procedures. Interactive educational materials, virtual reality simulations, and multimedia presentations help patients better understand their condition, treatment options, and expected outcomes, empowering them to make informed decisions about their care. The adoption of predictive analytics and artificial intelligence (AI) algorithms has enabled healthcare providers to identify patients who are at high risk of urinary incontinence recurrence or complications following artificial urinary sphincter implantation. By leveraging big data and machine learning techniques, healthcare providers can tailor treatment plans and postoperative management strategies to individual patient needs, thereby optimizing clinical outcomes and reducing healthcare costs. Advancements in healthcare technology are driving the growth of the North America Male Artificial Urinary Sphincter Market by improving the design and functionality of artificial urinary sphincters, enhancing surgical techniques, and integrating digital health solutions. These technological innovations enhance the efficacy, safety, and accessibility of artificial urinary sphincter procedures, leading to improved patient outcomes and greater market expansion. As healthcare technology continues to evolve, the male artificial urinary sphincter market is poised for further growth, offering opportunities for manufacturers, healthcare providers, and other stakeholders in the healthcare industry.
Rising Awareness and Education
Rising awareness and education play a critical role in driving the growth of the North America Male Artificial Urinary Sphincter Market. This heightened awareness encompasses various stakeholders, including patients, healthcare professionals, medical associations, advocacy groups, and healthcare organizations. Through educational initiatives, awareness campaigns, and information dissemination efforts, these stakeholders contribute to increasing understanding of urinary incontinence, available treatment options, and the benefits of artificial urinary sphincters, thus fostering market expansion. One of the primary drivers of rising awareness is the growing recognition of urinary incontinence as a prevalent and significant health issue among the general population. With aging demographics and increasing life expectancy, the prevalence of urinary incontinence is on the rise, particularly among older adults. As individuals become more aware of the symptoms and consequences of urinary incontinence, they are more likely to seek medical assistance and explore treatment options, including artificial urinary sphincters.Healthcare professionals, particularly urologists and general practitioners, play a crucial role in raising awareness and educating patients about urinary incontinence and available treatment modalities. Through continuing medical education programs, conferences, and professional associations, healthcare professionals stay updated on the latest developments in the field of urology and urinary incontinence management. This knowledge enables them to accurately diagnose patients, recommend appropriate treatment options, and advocate for the use of artificial urinary sphincters when indicated. Medical associations and organizations are actively involved in disseminating treatment guidelines, best practices, and evidence-based recommendations related to urinary incontinence management. By providing resources and educational materials to healthcare professionals, these organizations ensure that practitioners are well-informed about the efficacy and benefits of artificial urinary sphincters, thus facilitating their adoption in clinical practice. Advocacy groups and patient support organizations play a crucial role in raising awareness about urinary incontinence and advocating for improved access to treatment options. Through awareness campaigns, public events, and community outreach initiatives, these groups aim to destigmatize urinary incontinence, encourage open dialogue about the condition, and empower individuals to seek help and support. By sharing personal stories, providing educational resources, and connecting patients with healthcare providers, advocacy groups facilitate early detection, diagnosis, and treatment of urinary incontinence, including the use of artificial urinary sphincters when appropriate.
The advent of digital platforms and online resources has also contributed significantly to raising awareness about urinary incontinence and treatment options. Medical websites, social media platforms, online forums, and patient support groups provide valuable information, resources, and peer support to individuals affected by urinary incontinence. Patients can access educational materials, research articles, and testimonials from others who have undergone treatment with artificial urinary sphincters, empowering them to make informed decisions about their healthcare. Also, increasing awareness and education efforts encourage open communication between patients and healthcare providers, leading to earlier detection and intervention for urinary incontinence. Patients who are more informed about their condition and treatment options are more likely to proactively discuss their symptoms with their healthcare providers and seek appropriate care. This proactive approach facilitates timely diagnosis, treatment initiation, and follow-up care, ultimately improving patient outcomes and quality of life.
Reducing the stigma associated with urinary incontinence is another important aspect of rising awareness and education efforts. By raising awareness about the prevalence and impact of urinary incontinence, advocacy groups and healthcare organizations work to dispel myths and misconceptions surrounding the condition. This societal shift towards greater acceptance and understanding encourages individuals to seek help for urinary incontinence without fear of judgment or shame, thus increasing the demand for treatment options such as artificial urinary sphincters. Rising awareness and education drive the growth of the North America Male Artificial Urinary Sphincter Market by increasing understanding of urinary incontinence, treatment options, and the benefits of artificial urinary sphincters among patients, healthcare professionals, and the general public. Through educational initiatives, advocacy efforts, and information dissemination, stakeholders collaborate to destigmatize urinary incontinence, empower patients to seek help, and facilitate timely diagnosis and treatment. As awareness continues to grow, the demand for artificial urinary sphincters is expected to increase, thereby driving market expansion and improving patient outcomes.
Reimbursement and Insurance Coverage
Reimbursement and insurance coverage are significant drivers of growth in the North America Male Artificial Urinary Sphincter Market. These factors directly impact the accessibility, affordability, and utilization of artificial urinary sphincter procedures, making them more attractive options for both patients and healthcare providers. By mitigating financial barriers and incentivizing healthcare facilities to offer these procedures, reimbursement and insurance coverage contribute to market expansion and the adoption of artificial urinary sphincters as a standard of care for managing urinary incontinence. One of the primary ways in which reimbursement and insurance coverage drive market growth is by making artificial urinary sphincter procedures more affordable for patients. Urinary incontinence can significantly impact patients' quality of life and daily activities, leading many individuals to seek treatment options that offer long-term relief. However, the cost of surgical interventions such as artificial urinary sphincter implantation can be prohibitive for some patients, particularly those without adequate insurance coverage or financial resources. Reimbursement from insurance providers helps offset these costs, making artificial urinary sphincter procedures more accessible to a broader patient population.Insurance coverage for artificial urinary sphincter procedures encourages more patients to explore treatment options and undergo surgical intervention for urinary incontinence. When patients know that their insurance will cover the cost of the procedure, they are more likely to consider artificial urinary sphincters as a viable treatment option and pursue timely intervention for their condition. This increased demand for artificial urinary sphincter procedures translates into higher utilization rates and market growth. Healthcare providers, including urologists, hospitals, and ambulatory surgery centers, also benefit from reimbursement and insurance coverage for artificial urinary sphincter procedures. When insurance providers reimburse healthcare facilities for these procedures, it creates a financial incentive for providers to offer and promote artificial urinary sphincters as part of their service offerings. This, in turn, increases the availability and accessibility of artificial urinary sphincter procedures for patients, leading to higher patient volumes and revenue streams for healthcare facilities.
Reimbursement from insurance providers often comes with specific coding and billing requirements that healthcare facilities must adhere to in order to receive payment. Compliance with these requirements ensures that healthcare facilities maintain regulatory compliance and adhere to recognized medical standards when performing artificial urinary sphincter procedures. This helps uphold the quality and safety of care delivered to patients, enhancing the reputation of healthcare providers and fostering trust among patients and referring physicians. In addition to direct reimbursement for artificial urinary sphincter procedures, insurance coverage may also extend to related healthcare services, such as preoperative consultations, diagnostic tests, postoperative care, and device maintenance. Comprehensive insurance coverage for these ancillary services ensures that patients receive holistic care throughout the treatment process, from initial evaluation to long-term follow-up. This comprehensive coverage further enhances the value proposition of artificial urinary sphincter procedures for patients and healthcare providers alike, driving market growth. Reimbursement and insurance coverage for artificial urinary sphincter procedures often come with negotiated payment rates and contractual agreements between insurance providers and healthcare facilities. These payment rates may vary depending on factors such as geographic location, provider specialty, and patient demographics. Healthcare facilities that negotiate favorable payment rates with insurance providers stand to benefit from higher reimbursement rates and increased profitability for artificial urinary sphincter procedures. This financial incentive encourages healthcare facilities to invest in the necessary infrastructure, resources, and expertise to perform these procedures efficiently and effectively, further driving market growth.
Also, the presence of reimbursement and insurance coverage for artificial urinary sphincter procedures is often linked to regulatory approval and endorsement by professional medical societies and organizations. Insurance providers typically require evidence of clinical efficacy, safety, and cost-effectiveness before providing coverage for new medical technologies and procedures. Therefore, healthcare manufacturers and providers invest in clinical research, evidence generation, and outcomes studies to demonstrate the value proposition of artificial urinary sphincters and secure reimbursement from insurance providers. This evidence-based approach strengthens the market position of artificial urinary sphincters and promotes their widespread adoption as a standard of care for managing urinary incontinence. Reimbursement and insurance coverage are key drivers of growth in the North America Male Artificial Urinary Sphincter Market. These factors increase the affordability, accessibility, and utilization of artificial urinary sphincter procedures for patients, while providing financial incentives for healthcare providers to offer and promote these procedures. By mitigating financial barriers, ensuring regulatory compliance, and incentivizing investment in quality care, reimbursement and insurance coverage contribute to market expansion and the adoption of artificial urinary sphincters as an integral component of urinary incontinence management.
Key Market Challenges
High Procedure Costs
Male artificial urinary sphincter implantation is a surgical procedure that can be costly. The expense includes the cost of the device itself, surgical fees, anesthesia, and post-operative care.For many patients, the high cost of the procedure can be a significant barrier to access. Even with insurance coverage, out-of-pocket expenses can be substantial. This financial constraint can deter individuals from seeking treatment, slowing down market growth. In some regions, insurance providers may not fully cover the cost of male artificial urinary sphincter procedures, leaving patients with a considerable financial burden. Reduced insurance coverage can impede the market's expansion by limiting patient access.
Stigma and Awareness
Male urinary incontinence is often associated with a certain level of social stigma. This stigma can prevent individuals from discussing their condition with healthcare professionals, leading to delayed diagnosis and treatment.Despite efforts to raise awareness, many individuals, including healthcare providers, may still have limited knowledge about male urinary incontinence and the available treatment options. Lack of awareness can hinder early intervention and impede market growth. Patients may fear disclosing their condition due to embarrassment or concerns about societal perception. This fear can lead to delayed treatment seeking, impacting the market's growth.
Complications and Revision Surgeries
While male artificial urinary sphincters are generally effective, complications can arise. These may include device erosion, infection, mechanical failure, or the need for device revisions. Complications can result in additional healthcare costs and reduce patient satisfaction.The necessity of revision surgeries can pose challenges, as they are associated with additional costs, recovery time, and potential complications. Patients may be discouraged by the prospect of revision surgeries, and this can influence their decision to undergo the initial implantation procedure. Managing complications and revisions requires a high level of clinical expertise. Ensuring that healthcare providers have the necessary skills to handle such cases can be a challenge and may impact market growth.
Key Market Trends
Minimally Invasive Procedures
One of the most prominent trends in the male artificial urinary sphincter market is the increasing adoption of minimally invasive procedures for implanting these devices.Minimally invasive techniques involve smaller incisions, reduced tissue damage, and shorter recovery times. This trend is driven by the desire to minimize patient discomfort and enhance the overall patient experience. As minimally invasive procedures become more widely adopted, they are likely to attract more patients who are hesitant about undergoing surgery. This trend contributes to the market's growth by expanding the pool of potential candidates for male artificial urinary sphincter implantation.
Personalized Treatment
Personalization and customization of male artificial urinary sphincter treatment plans are gaining prominence. Patients have unique needs, and personalized treatment allows healthcare providers to tailor the device settings to suit individual requirements. This trend recognizes that a one-size-fits-all approach may not be optimal for every patient. Personalized treatment not only enhances patient satisfaction but also improves treatment outcomes. Healthcare providers are more likely to recommend male artificial urinary sphincters when they can be personalized to meet the specific needs of each patient. As a result, the market is growing as a wider range of patients opt for these personalized treatment options.Segmental Insights
Type Insights
Based on Type, the AUS with a balloon reservoir (3-component) segment emerged as the dominant player in the North America market for Male Artificial Urinary Sphincter in 2023. AUS with a balloon reservoir, categorized as a 3-component system, is highly regarded for its effectiveness in managing male urinary incontinence. These devices are designed to mimic the natural sphincter's functioning, providing users with better control over their urinary flow. The three-component design, which typically includes a cuff, a pressure-regulating balloon, and a control pump, ensures precise control and minimizes leakage.The balloon reservoir within the 3-component AUS offers a comfortable and discreet solution for patients. Its design ensures that the device remains compact and unobtrusive, contributing to greater patient comfort and ease of use. AUS with balloon reservoirs have garnered strong clinical endorsement and have been recognized as a reliable treatment option for male urinary incontinence. The effectiveness of this 3-component design is often backed by medical professionals, increasing patient trust and adoption.
The 3-component AUS allows for individualized adjustments. Physicians can tailor the device to the specific needs of each patient, ensuring optimal performance. This level of customization ensures a higher success rate and patient satisfaction. AUS devices with a balloon reservoir are known for their durability and longevity. This feature is particularly attractive to patients as it reduces the need for frequent replacements or adjustments. These factors are expected to drive the growth of this segment.
End User Insights
The hospitals segment is projected to experience rapid growth during the forecast period. Hospitals are hubs of clinical expertise, with urologists and other specialists who can accurately diagnose and recommend treatment for male urinary incontinence. The urological conditions that require artificial urinary sphincters often necessitate specialized care, which is readily available in hospital settings. This makes hospitals the primary point of contact for patients seeking treatment for their condition.The implantation of male artificial urinary sphincters is a surgical procedure that demands specialized skills and resources. Hospitals are equipped with state-of-the-art surgical facilities and trained surgical teams capable of performing these complex procedures with a high degree of precision and safety. The sterile environments, advanced technology, and support staff available in hospitals ensure the successful implantation of artificial urinary sphincters. Post-surgical care is essential for patients who have undergone artificial urinary sphincter implantation. Hospitals provide comprehensive post-operative care, monitoring patients for any complications, and offering immediate interventions if necessary. This ongoing care is vital for the long-term success of the procedure. Hospitals offer both inpatient and outpatient services, accommodating a wide range of patient needs. Some patients may require an overnight stay for surgery and recovery, while others can receive outpatient care. This flexibility makes hospitals accessible to a broad spectrum of patients, regardless of the severity of their condition. These factors collectively contribute to the growth of this segment.
Country Insights
The United States has emerged as the dominant country in the North America Male Artificial Urinary Sphincter (AUS) market in 2023, commanding the largest market share in terms of value. This leadership position is underpinned by several factors that collectively contribute to the country's prominence in this specialized healthcare sector. The United States grapples with a high prevalence of urinary incontinence in men, a condition that significantly impacts quality of life and necessitates effective treatment options such as the AUS. This widespread prevalence drives demand for AUS devices and procedures, positioning the United States as a key market for this medical intervention. The high disposable income of patients in the United States plays a pivotal role in driving market dominance. With a culture of prioritizing health and wellness, American patients are more willing and able to invest in advanced medical technologies and procedures to address urinary incontinence issues. This financial capacity enhances accessibility to AUS devices and procedures, further solidifying the United States' position as a dominant market player.The ready availability of AUS devices and procedures in the United States contributes to its market dominance. AUS devices are typically covered by insurance, making them accessible to a broad spectrum of patients. While patients may be responsible for some out-of-pocket costs, the widespread availability of insurance coverage mitigates financial barriers to access. The United States boasts a large pool of urologists with expertise in AUS implantation procedures, ensuring patients have access to skilled healthcare professionals for diagnosis, treatment, and follow-up care. AUS devices are readily available at most hospitals and surgery centers across the country, further facilitating patient access and enhancing market penetration.
Key Market Players
- Boston Scientific Corporation
- Rigicon, Inc.
- Teleflex Incorporated
- GE HealthCare Technologies, Inc.
- Health, Inc.
- Medline Industries, LP
- Henry Schein, Inc.
- Becton, Dickinson and Company
- Owens & Minor, Inc.
- Illumina, Inc.
Report Scope:
In this report, the North America Male Artificial Urinary Sphincter Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:North America Male Artificial Urinary Sphincter Market, By Type:
- AUS with a Balloon Reservoir (3-Component)
- AUS with a Spring (2-Component)
North America Male Artificial Urinary Sphincter Market, By End User:
- Hospitals
- Clinics & Other Health Centers
- Academic & Research Centers
North America Male Artificial Urinary Sphincter Market, By Country:
- United States
- Canada
- Mexico
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the North America Male Artificial Urinary Sphincter Market.Available Customizations:
North America Male Artificial Urinary Sphincter market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Boston Scientific Corporation
- Rigicon, Inc.
- Teleflex Incorporated
- GE HealthCare Technologies, Inc.
- Health, Inc.
- Medline Industries, LP
- Henry Schein, Inc.
- Becton, Dickinson and Company
- Owens & Minor, Inc.
- Illumina, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 130 |
| Published | August 2024 |
| Forecast Period | 2023 - 2029 |
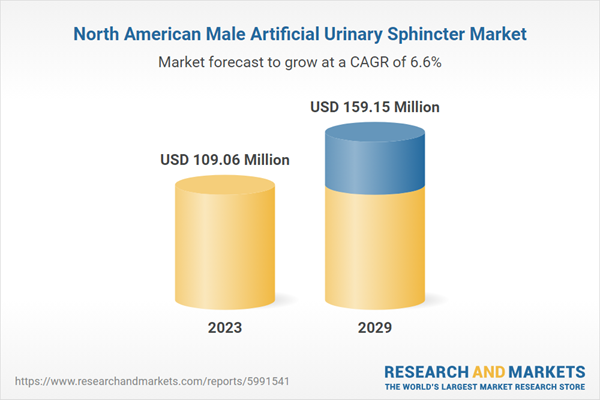
| Estimated Market Value ( USD | $ 109.06 Million |
| Forecasted Market Value ( USD | $ 159.15 Million |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |