Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Prevalence of Atopic Dermatitis
The rising incidence of atopic dermatitis directly expands the patient population requiring therapeutic interventions. As more individuals are diagnosed with AD, there is a greater demand for effective treatment options. The prevalence of atopic dermatitis (AD) in Japan is estimated to range from 5% to 32% among children aged 6 months to under 18 years, and from 2% to 10% among adults. This broader patient base necessitates an increase in the availability and variety of therapeutics, driving market growth. The increased prevalence translates into a larger target market for pharmaceutical companies and healthcare providers, prompting investment in new treatments and management strategies. The growing prevalence of AD creates a heightened need for innovative and effective therapies. With a larger number of patients seeking relief from symptoms, there is increased pressure on the market to develop and offer advanced therapeutic options. This demand drives pharmaceutical companies to focus on research and development, resulting in the introduction of novel treatments such as biologics and targeted therapies. The push for innovation is fueled by the need to address the diverse and severe manifestations of AD observed in a growing patient population.A higher prevalence of atopic dermatitis leads to increased healthcare utilization, including more frequent doctor visits, diagnostic tests, and therapeutic interventions. As patients seek medical care and management for their condition, healthcare costs rise, creating a substantial market for AD therapeutics. The economic burden of managing a larger patient population, including direct costs of treatments and indirect costs related to reduced quality of life and productivity, drives investment and focus in the market. The growing prevalence of AD provides ample opportunities for clinical research and trials. A larger patient pool enables researchers to conduct more extensive studies and gather data on various treatment approaches. This expanded research base contributes to the development of new therapies and enhances understanding of the disease, ultimately driving market growth. Pharmaceutical companies are incentivized to explore new treatment modalities and participate in clinical trials to address the needs of a growing patient population.
The increasing prevalence of atopic dermatitis heightens the market potential for therapeutics, attracting investment from both domestic and international pharmaceutical companies. Investors and stakeholders recognize the substantial market opportunity presented by a larger patient base and higher demand for treatments. This investment drives the development of new drugs, improves existing treatments, and supports market expansion. The Japanese government and healthcare system are likely to respond to the rising prevalence of atopic dermatitis by implementing policies and programs aimed at improving disease management. This response may include increased funding for research, changes in reimbursement policies, and initiatives to enhance patient access to therapies. Such actions support the growth of the therapeutics market by facilitating the availability and adoption of effective treatments.
Advances in Therapeutic Technologies
Advances in therapeutic technologies are a major driver of growth in the Japan Atopic Dermatitis (AD) therapeutics market. These technological innovations impact various aspects of the market, enhancing treatment efficacy, patient outcomes, and overall market dynamics. One of the most significant technological advancements in the treatment of atopic dermatitis is the development of biologic therapies. These advanced treatments, such as monoclonal antibodies, specifically target immune system pathways involved in AD, offering more precise and effective management of the condition. For instance, biologics like dupilumab inhibit interleukin-4 and interleukin-13, key cytokines involved in inflammation and allergic responses. The high efficacy and safety profiles of biologics have revolutionized the treatment landscape for severe atopic dermatitis, driving their adoption and contributing to market growth. Advances in drug delivery technologies have improved the administration and effectiveness of AD therapies. Innovations such as self-administered injectables and auto-injectors have made it easier for patients to receive biologic treatments at home, enhancing adherence and convenience. Additionally, improvements in topical formulations, including enhanced penetration technologies and controlled-release systems, allow for more effective delivery of therapeutic agents to the skin. These advancements in drug delivery systems increase patient compliance and optimize treatment outcomes, fueling market expansion.The emergence of targeted small molecules represents another key advancement in therapeutic technologies. Unlike traditional systemic treatments, these small molecules specifically inhibit certain pathways involved in the pathogenesis of atopic dermatitis. By targeting molecular mechanisms with high precision, these therapies offer improved efficacy and reduced side effects compared to older treatments. The introduction and adoption of these targeted therapies contribute to market growth by providing new, effective options for managing AD. Advances in therapeutic technologies have also led to the development of combination therapies that integrate multiple treatment modalities to enhance overall efficacy. For example, combining biologic therapies with topical treatments or other systemic medications can provide synergistic effects, leading to better management of atopic dermatitis symptoms. This approach not only improves treatment outcomes but also drives market growth by expanding the range of available therapeutic options and addressing various aspects of the disease.
Personalized medicine, enabled by advances in genomics and molecular diagnostics, is transforming the approach to treating atopic dermatitis. By tailoring treatments based on individual genetic profiles and disease characteristics, personalized medicine enhances the precision and effectiveness of therapies. This trend towards personalized treatment approaches is driving market growth by addressing the specific needs of patients and improving therapeutic outcomes. Technological advancements in diagnostic tools, such as more sophisticated imaging techniques and biomarkers, enhance the accuracy of atopic dermatitis diagnosis and disease monitoring. Early and accurate diagnosis allows for timely intervention and personalized treatment plans, leading to better management of the condition. The availability of advanced diagnostic tools supports the growth of the therapeutics market by facilitating the effective application of new treatments and improving patient outcomes.
Increasing Investment in Research and Development
Increasing investment in research and development (R&D) plays a pivotal role in driving the growth of the Japan Atopic Dermatitis (AD) therapeutics market. This investment fuels various aspects of the market by advancing therapeutic options, enhancing clinical outcomes, and fostering innovation. Increased R&D investment accelerates the discovery and development of novel therapeutic agents for atopic dermatitis. Pharmaceutical and biotechnology companies allocate substantial resources to explore new drug candidates, including biologics, small molecules, and advanced topical formulations. This focus on innovation results in the introduction of new therapies that offer improved efficacy, safety, and patient outcomes. As new treatments become available, they expand the market by providing more options for managing atopic dermatitis, addressing unmet medical needs, and appealing to both healthcare providers and patients. Investment in R&D not only fosters the development of new therapies but also supports the enhancement of existing treatments. Companies invest in refining and optimizing current therapeutic approaches to improve their effectiveness, reduce side effects, and enhance patient adherence. For example, advancements in drug delivery systems and formulation technologies can make existing treatments more effective and convenient for patients. These improvements contribute to market growth by increasing the value and attractiveness of existing therapeutic options.R&D investment drives progress in clinical research and trials, providing critical data on the safety and efficacy of new and existing treatments. Well-funded clinical trials enable the evaluation of innovative therapies in diverse patient populations and under various clinical conditions. Successful trials lead to regulatory approvals and market entry of new therapies. Additionally, robust clinical research supports the development of evidence-based treatment guidelines, enhancing the credibility and adoption of new therapies in clinical practice. This rigorous research environment contributes to market growth by ensuring that new treatments are safe, effective, and backed by strong clinical evidence. Increased R&D investment often leads to collaborations and partnerships between pharmaceutical companies, research institutions, and academic centers. These partnerships facilitate the pooling of expertise, resources, and knowledge, accelerating the development of new therapies and advancing scientific understanding of atopic dermatitis. Collaborative efforts can also streamline the drug development process and enhance the efficiency of bringing new treatments to market. Such partnerships and collaborations contribute to market growth by fostering innovation and expanding the therapeutic landscape.
High levels of R&D investment signal a robust and dynamic market, attracting additional investment from venture capitalists, private equity firms, and other financial stakeholders. The influx of capital supports further research, development, and commercialization of therapeutic products. As the market grows and evolves, it becomes increasingly attractive to investors, creating a positive feedback loop that drives continued investment and innovation. R&D investments are critical in addressing unmet medical needs within the atopic dermatitis patient population. By focusing on understanding the underlying mechanisms of the disease and developing targeted therapies, investments in R&D can lead to breakthroughs in treatment and management. Addressing these unmet needs enhances patient outcomes and drives market growth by expanding the range of available therapeutic options.
Key Market Challenges
High Cost of Advanced Therapies
The cost associated with advanced therapeutic options, particularly biologics, is a significant barrier to market growth. Biologic therapies, which offer targeted and effective treatment for severe atopic dermatitis, often come with high price tags due to their complex manufacturing processes and development costs. These high costs can limit patient access, especially in a healthcare system that is increasingly focused on cost containment and budget management. While there are efforts to negotiate pricing and provide patient assistance programs, the overall affordability and accessibility of these advanced therapies remain a challenge, potentially hindering widespread adoption and market expansion.Limited Awareness and Understanding of AD
Despite increasing awareness, there remains a significant gap in public and clinical understanding of atopic dermatitis, particularly regarding its management and available treatment options. This limited awareness can lead to delayed diagnosis and treatment, impacting patient outcomes and market growth. Additionally, healthcare professionals may not always be up to date with the latest advancements in AD therapeutics, which can affect the adoption of newer, more effective treatments. Efforts to enhance education and awareness among both patients and healthcare providers are ongoing but addressing this gap remains a challenge for driving market growth.Regulatory and Reimbursement Hurdles
The regulatory environment and reimbursement policies can pose challenges for the growth of the AD therapeutics market. Obtaining regulatory approval for new therapies can be a lengthy and complex process, which may delay the availability of innovative treatments. Additionally, securing reimbursement and favorable pricing from public and private payers can be challenging, particularly for high-cost therapies. Variations in reimbursement policies and the need for extensive clinical evidence to support cost-effectiveness further complicate market entry and expansion. These regulatory and reimbursement hurdles can restrict the availability of new treatments and slow the overall market growth.Key Market Trends
Increasing Adoption of Biologic Therapies
The most significant trends in the Japan AD therapeutics market is the growing adoption of biologic therapies. These advanced treatments, which include monoclonal antibodies and targeted cytokine inhibitors, offer precise mechanisms for managing severe atopic dermatitis. Biologics such as dupilumab have demonstrated substantial efficacy in reducing inflammation and improving skin symptoms in patients who have not responded adequately to traditional treatments. The continued development and approval of new biologics, combined with their ability to provide long-term relief and better safety profiles, are likely to drive their increased uptake. This trend is bolstered by ongoing research and clinical trials that continue to validate the effectiveness of biologics, as well as by supportive reimbursement policies that facilitate patient access to these advanced therapies.Growth of Digital Health Solutions and Remote Monitoring
Another crucial trend influencing the market is the integration of digital health solutions and remote monitoring technologies. The rise of digital health tools, such as telemedicine platforms and mobile health applications, allows for enhanced patient management and real-time monitoring of atopic dermatitis. These technologies facilitate more personalized treatment plans, improved patient engagement, and better adherence to therapeutic regimens. For instance, digital tools can track disease progression and treatment responses, enabling healthcare providers to make more informed decisions and adjust therapies as needed. The growing acceptance of digital health solutions in Japan, driven by technological advancements and the need for more efficient healthcare delivery, is expected to contribute significantly to the market's growth.Rising Awareness and Patient Education
The increased awareness and education surrounding atopic dermatitis are also playing a pivotal role in the market's expansion. As public and medical awareness of AD grows, patients are becoming more informed about the condition and available treatment options. Educational campaigns, improved access to information, and patient advocacy initiatives are helping to demystify the disease and promote early diagnosis and treatment. This heightened awareness is leading to greater demand for effective therapies and more proactive management of the condition. Additionally, awareness programs that emphasize the importance of adhering to treatment plans and managing lifestyle factors associated with AD are further driving the market's growth by encouraging patients to seek and adhere to appropriate therapies.Segmental Insights
Drug Class Insights
Based on Drug Class, the Biologic Therapy segment emerged as the dominant in the market for Japan Atopic Dermatitis Therapeutics in 2024. Biologic therapies, such as monoclonal antibodies and cytokine inhibitors, offer a high degree of precision in targeting the underlying pathophysiology of atopic dermatitis. For instance, drugs like dupilumab, which inhibits interleukin-4 and interleukin-13 signaling, have shown robust efficacy in reducing the symptoms and severity of AD. This targeted approach not only improves patient outcomes but also enhances the safety profile by minimizing off-target effects compared to systemic immunosuppressants. Japan has witnessed a growing prevalence of atopic dermatitis, leading to an increased demand for advanced therapeutic options. Traditional treatments, such as topical steroids and calcineurin inhibitors, while effective, often come with limitations related to long-term use and potential side effects. Biologic therapies address this unmet need by providing a novel mechanism of action that offers a more sustained therapeutic benefit and improved quality of life for patients who have not responded adequately to conventional treatments.The dominance of biologic therapies is also driven by significant investments in research and development. Japanese pharmaceutical companies and international players have heavily invested in developing biologics, leading to a rich pipeline of innovative therapies. This focus on R&D has accelerated the availability of advanced biologics and cemented their position as a leading choice in the market. The Japanese healthcare system has increasingly recognized the value of biologic therapies in managing chronic and severe forms of atopic dermatitis. Reimbursement policies have evolved to support the inclusion of these advanced treatments, facilitating greater market penetration. As a result, biologic therapies have become more accessible to patients and healthcare providers, further reinforcing their dominance in the therapeutic landscape. The strong clinical evidence supporting the efficacy and safety of biologic therapies has garnered favorable regulatory approvals and endorsements. Regulatory bodies in Japan have granted expedited approval pathways for several biologics, reflecting their critical role in addressing severe atopic dermatitis. This regulatory support underscores the importance of biologic therapies in the market and encourages continued investment and development in this segment. These factors are expected to drive the growth of this segment.
Route of Administration Insights
The injectable segment is projected to experience rapid growth during the forecast period. Injectables, such as subcutaneous or intravenous biologics, offer robust and rapid therapeutic efficacy compared to other routes of administration. These medications, including monoclonal antibodies and other biologics, often provide faster relief from severe symptoms of atopic dermatitis. For instance, drugs like dupilumab and tralokinumab are administered via injection and have demonstrated high efficacy in reducing inflammation and improving skin conditions. The rapid onset of action is a key factor driving their dominance in the market. For patients with moderate to severe atopic dermatitis, injectables offer a significant advantage in managing chronic and complex cases. The injectable formulations often provide a long-acting effect, which translates into fewer dosing frequencies and improved adherence compared to oral medications or topical treatments. This long-acting nature simplifies disease management for patients and healthcare providers, reinforcing the dominance of injectables. Injectables in the atopic dermatitis market are increasingly designed for convenience, such as self-administration options. Advances in injection technology, including pre-filled pens and auto-injectors, have made it easier for patients to administer their medications at home, improving adherence rates and patient satisfaction. This ease of use contributes to the segment’s growing dominance.The injectable segment benefits from strong clinical evidence supporting its efficacy and safety. Regulatory bodies in Japan have approved various injectable treatments based on extensive clinical trials demonstrating their benefits in managing severe atopic dermatitis. This regulatory endorsement fosters trust in injectable therapies and encourages their adoption within the market. Significant investment in the development of injectable therapies reflects the growing recognition of their value in treating atopic dermatitis. Pharmaceutical companies have concentrated their research and development efforts on enhancing injectable formulations, which has led to the introduction of new and innovative products in the market. This focus on injectables has reinforced their dominance and expanded their market share. The injectable segment’s dominance is also driven by its competitive advantage over other routes of administration. Injectables often provide a more controlled and targeted delivery of therapeutic agents, leading to more effective management of atopic dermatitis symptoms. This competitive edge enhances the segment's appeal to both healthcare providers and patients. These factors collectively contribute to the growth of this segment.
Regional Insights
Kanto emerged as the dominant in the Japan Atopic Dermatitis Therapeutics market in 2024, holding the largest market share in terms of value. The Kanto Region's dominance in the Japan Atopic Dermatitis (AD) therapeutics market can be attributed to several key factors that influence both the supply and demand dynamics within this geographic area. This region, which includes major urban centers such as Tokyo and Yokohama, exhibits characteristics that significantly contribute to its leading position in the market. The Kanto Region is Japan’s most populous area, with a dense population that includes a substantial number of individuals affected by atopic dermatitis. The high prevalence of AD in this region drives demand for therapeutic interventions. Urban areas with large populations often experience higher rates of chronic conditions like AD, leading to greater healthcare utilization and market activity.The Kanto Region boasts a highly developed healthcare infrastructure, including leading hospitals, specialized clinics, and research facilities. This advanced infrastructure supports the availability and accessibility of cutting-edge therapies for atopic dermatitis. High-quality healthcare services and specialized dermatology centers in the region contribute to the widespread adoption of new and advanced treatments. The Kanto Region is a hub for pharmaceutical and biotechnology companies conducting research and development (R&D) in dermatology and atopic dermatitis treatments. The presence of major pharmaceutical firms and research institutions fosters innovation and accelerates the development of new therapeutics. This concentration of R&D activities enhances the region’s role in the introduction and uptake of novel therapies.
The Kanto Region, with its economic affluence and high disposable income levels, has higher healthcare spending compared to other regions. Residents in this region are more likely to invest in advanced and often costly treatments for atopic dermatitis. This economic advantage supports the market for premium and innovative therapeutics, driving growth and dominance. Major pharmaceutical companies with a strong presence in the Kanto Region are key players in the AD therapeutics market. Their operations, including marketing and distribution, are centered in this area, ensuring that new treatments are readily available. These companies often launch their latest products and therapies in the Kanto Region first, capitalizing on its significant market potential.
Key Market Players
- Otsuka Holdings Co., Ltd.
- Maruho Co., Ltd
- Taisho Pharmaceutical Holdings
- KAKEN PHARMACEUTICAL CO., LTD.
- FUJIFILM Corporation
- Mitsubishi Tanabe Pharma Corporation
- Sanofi
- Novartis AG
- AbbVie Inc.
- LEO Pharma A/S
Report Scope:
In this report, the Japan Atopic Dermatitis Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Japan Atopic Dermatitis Therapeutics Market, By Drug Class:
- Corticosteroids
- Calcineurin Inhibitors
- Immunosuppressants
- Biologic Therapy
- PDE-4 Inhibitor
- Antibiotics
- Antihistamines
- Emollients
Japan Atopic Dermatitis Therapeutics Market, By Route of Administration:
- Topic
- Oral
- Injectable
Japan Atopic Dermatitis Therapeutics Market, By Distribution Channel:
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Dermatology Clinics
- Others
Japan Atopic Dermatitis Therapeutics Market, By Region:
- Hokkaido
- Tohoku
- Kanto
- Chubu
- Kansai
- Chugoku
- Shikoku
- Kyushu
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Japan Atopic Dermatitis Therapeutics Market.Available Customizations:
Japan Atopic Dermatitis Therapeutics market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Otsuka Holdings Co., Ltd.
- Maruho Co., Ltd
- Taisho Pharmaceutical Holdings
- KAKEN PHARMACEUTICAL CO., LTD.
- FUJIFILM Corporation
- Mitsubishi Tanabe Pharma Corporation
- Sanofi
- Novartis AG
- AbbVie Inc.
- LEO Pharma A/S
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 82 |
| Published | August 2024 |
| Forecast Period | 2024 - 2030 |
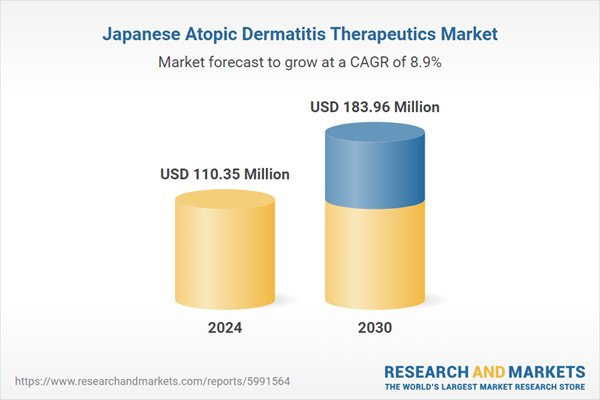
| Estimated Market Value ( USD | $ 110.35 Million |
| Forecasted Market Value ( USD | $ 183.96 Million |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 10 |