Patent Landscape Report Coverage
This report offers a comprehensive understanding of the global hemostats patent landscape. It covers trends in patent filings, significant technological advancements, and emerging innovations in the hemostats sector. The report includes detailed information on patent filings, grants, and the leading companies driving progress in hemostatic technologies. By highlighting strategic developments and advanced technologies, this report is an invaluable resource for stakeholders seeking to understand the intellectual property dynamics and competitive landscape of the hemostats industry.Global Hemostat Patent Outlook
- The global hemostats patent landscape is fueled by biocompatible materials and novel formulations, with over 5,000 filed. Advancements aim to enhance hemostatic efficacy, minimise adverse reactions, and promote natural healing processes. The integration of hemostats with minimally invasive and robotic surgical techniques further fuels innovation.
- Ethicon Inc., Tepha Inc., and Tyco Healthcare are leading companies in the hemostatic technology industry, filing numerous patents for bio-resorbable materials and surgical solutions. Their focus on patient safety and surgical outcomes is driving significant patent activity, positioning them as key players in the industry's future.
- The US leads the patent landscape for hemostats with over 5,500 patents, focusing on advanced materials and regulatory compliance. Europe, particularly Germany and the UK, contributes 3,500 patents, focusing on safety and surgical standards. The Asia-Pacific region, led by China and Japan, is emerging with over 3,000 patents, driven by cost-effective and scalable hemostatic solutions.
Hemostat: Introduction
Hemostats are critical tools in surgical procedures, designed to achieve hemostasis by promoting the clotting of blood. These devices are used to control bleeding during surgery, facilitating quicker recovery and reducing the risk of complications. The patent landscape for hemostats is driven by the need for more effective hemostatic agents, integration with advanced surgical techniques, and development of bio-compatible materials.- The advancement of biocompatible materials is essential for enhancing the safety and effectiveness of hemostats. Over 5,000 patents focus on the development of these advanced materials, which are designed to enhance hemostatic efficacy and minimise adverse reactions. These materials are crucial in reducing inflammation and promoting natural healing processes post-surgery.
- The integration of hemostats with modern surgical technologies is a key focus area. Approximately 4,200 patents emphasize the integration of hemostats with robotic and minimally invasive surgical techniques. This integration enhances precision and effectiveness, ensuring rapid hemostasis in complex surgical environments and improving overall surgical outcomes.
- The development of novel hemostatic formulations is critical for improving patient outcomes. Around 3,800 patents focus on these advanced formulations that offer rapid action and a longer duration of effect. These innovations ensure reliable bleeding control during and after surgical procedures, enhancing patient safety and recovery.
Global Hemostats Patent Segmentation Analysis
The report provides an in-depth analysis of the patents in this field by the following segmentation:
Analysis by Product Type
- Combination
- Oxidized Regenerated Cellulose Based
- Gelatine Based
- Collagen Based Hemostat
Analysis by Instrument Type
- Halstead Mosquito Hemostatic Forceps
- Kelly And Crile Hemostatic Forceps
- Rochester-Carmalt Hemostatic Forceps
Analysis by Formulation
- Matrix and Gel Hemostats
- Sheet and Pad Hemostats
- Sponge Hemostats
- Powder Hemostats
Analysis by Application
- Orthopaedic
- General surgery
- Neurological surgery
- Cardiovascular surgery
- Reconstructive surgery
- Gynaecological surgery
Analysis by End User
- Ambulatory Centers
- Hospitals
- Clinics
- Community Healthcare
- Others
Hemostats Patent Jurisdiction Analysis
The global patent landscape for hemostats exhibits diverse regional focuses and innovations. In North America, particularly the United States, over 5,500 patents emphasize the development of advanced hemostatic materials and surgical techniques. European countries, such as Germany and the UK, hold over 3,500 patents, focusing on regulatory compliance and safety standards. The Asia-Pacific region, led by China and Japan, has filed over 3,000 patents, driven by the demand for cost-effective and efficient hemostatic solutions to support expanding healthcare systems.Patent Profile of Key Companies
The patent landscape for hemostats is shaped by several key companies driving innovation and securing intellectual property. Here is an overview of their patent activities:Ethicon Inc
Ethicon Inc., based in Somerville, New Jersey, holds over 1,800 patents related to hemostatic devices, with approximately 200 patents in progress. The company focuses on innovations in biocompatible materials and advanced formulations, enhancing hemostatic efficacy and patient safety.Tepha Inc
Headquartered in Lexington, Massachusetts, Tepha Inc. has filed over 1,500 patents in the hemostat sector, with around 150 patents currently in progress. The company specialises in developing bio-resorbable materials that offer effective hemostasis and promote natural healing processes.Tyco Healthcare
Tyco Healthcare, located in Mansfield, Massachusetts, holds over 1,600 patents related to hemostats, with approximately 180 patents in progress. The company focuses on innovative hemostatic solutions that integrate seamlessly with modern surgical practices, improving surgical outcomes and efficiency.Other key players in the industry include Omrix Biopharmaceuticals Inc. and Joshua R&d Technologies LLc.
Key Questions Answered in the Global Hemostats Patent Landscape Report
- What are the key drivers of increasing patent activitiesin hemostatic technology?
- How do patent trends vary across different regions in the hemostat landscape?
- Which companies are leading in patent filings for hemostats?
- What technological advancements are shaping the patent landscape of hemostatic solutions?
- How is the demand for biocompatible materials influencing patent activity?
- What challenges are faced by patent landscapein the development and implementation of hemostatic devices?
- How is regulatory compliance impacting the patent landscape for these devices?
- How are patents addressing the integration of hemostats with surgical techniques?
- What are the trends in patent filings for product and instrument types?
- How is the Asia-Pacific region contributing to the hemostat patent landscape?
- What innovations are being developed to enhance surgical outcomes and safety?
- How are hospitals and surgical centers driving patent activity in hemostatic technology?
Reasons to Purchase this Report
This report offers an in-depth analysis of the patent landscape, covering key trends, technological advancements, and regional insights. It provides detailed segmentation and highlights areas of significant innovation and activity. By examining leading companies' strategies and patent portfolios, the report elucidates competitive dynamics and emerging opportunities. Stakeholders will gain valuable information for strategic decision-making, ensuring they stay ahead in the evolving landscape. This comprehensive coverage makes it an essential resource for understanding the industry's future direction.This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Ethicon Inc
- Tyco Healthcare
- Tepha Inc
- Omrix Biopharmaceuticals Inc
- Joshua R&d Technologies LLC
- Covidien Lp
- Human Genome Sciences Inc
- Ecolab Usa Inc
- Medtronic Inc
- Baxter Int
- Cilag Gmbh Int
- Boston Scient Scimed Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2024 |
| Forecast Period | 2024 - 2032 |
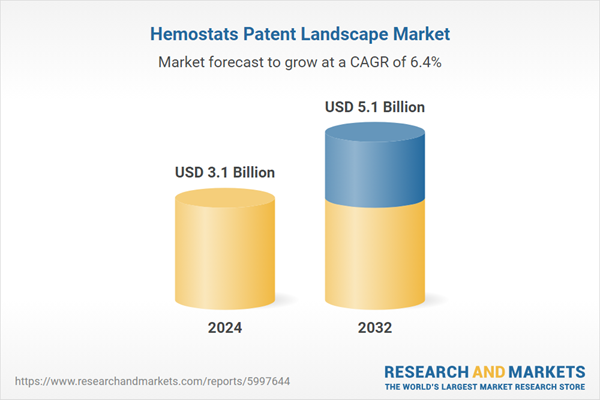
| Estimated Market Value ( USD | $ 3.1 Billion |
| Forecasted Market Value ( USD | $ 5.1 Billion |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |