Patent Landscape Report Coverage
The patent analysis report for the aortic stent graft landscape provides comprehensive coverage, highlighting innovations, key patents, and emerging technologies. It examines patent trends, assesses the competitive landscape, and identifies leading entities driving advancements in stent graft technology. The report also delves into patent filing activities, geographical distribution, and collaboration patterns, offering a nuanced understanding of the technological progression and strategic initiatives within the sector. By analysing patent portfolios and their impact, the report equips stakeholders with crucial insights for informed decision-making and strategic planning.Global Aortic Stent Graft Patent Outlook
- Cutting-edge advancements in novel polymers, metal alloys, and bioengineered coatings are propelling significant patent filings. Customisable stent graft designs that cater to individual patient anatomies are also spurring innovation, with over 200 patents focusing on material innovations and bioengineered solutions.
- Leading the charge in patent activity, companies like Cook Medical Technologies LLC, Boston Scientific Scimed Inc., and Edwards Lifesciences Corp hold substantial patent portfolios. These companies are continuously innovating, with hundreds of patents filed for state-of-the-art deployment mechanisms and patient-specific graft designs.
- The United States leads with over 600 patents, driven by robust R&D and strong patent laws. Europe, with 500 patents, focuses on biocompatible materials and customisation. Asia-Pacific, advancing with 450 patents, benefits from expanding healthcare domain and increased investment in cutting-edge stent graft technologies.
Aortic Stent Graft Introduction
Aortic stent grafts are medical devices used to reinforce weakened sections of the aorta, particularly in cases of aneurysms. These devices are essential in endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR) procedures. They consist of a fabric-covered stent that is inserted into the aorta via a minimally invasive procedure, providing structural support and reducing the risk of aortic rupture. The development of these devices focuses on improving durability, flexibility, and ease of deployment to enhance patient outcomes.- Patents focus on the development of advanced materials such as novel polymers and metal alloys to improve the durability and flexibility of aortic stent grafts. For example, devices using Gore-Tex material or Nitinol alloys have over 200 patents filed for material innovations, enhancing the longevity and adaptability of the stent grafts.
- Recent patents emphasize the use of bioengineered coatings that promote endothelialisation and reduce the risk of thrombosis. Innovations in coatings, such as heparin-coated stents and drug-eluting technologies, highlight over 160 patents focusing on these advancements, aiming to enhance biocompatibility and the long-term success of the stent grafts.
- Patents for customisable and patient-specific aortic stent graft designs are critical for improving patient outcomes. Innovations like the EXCLUDER® AAA Endoprosthesis feature customisable components to fit individual patient anatomies, with over 180 patents filed for technologies that enable tailored stent grafts, ensuring better compatibility and effectiveness.
Global Aortic Stent Graft Patent Segmentation Analysis
The report provides an in-depth analysis of the patents in this field by the following segmentation:
Analysis by Product Type
- Abdominal Aortic Stent Graft
- Thoracic Aortic Stent Graft
Analysis by End User
- Hospitals
- Ambulatory Surgical Centers
- Others
Aortic Stent Graft Patent Jurisdiction Analysis
The global patent landscape for aortic stent grafts is evolving, with notable activity in the US, Europe, and Asia-Pacific.- The United States leads with over 600 patents filed historically and 250 currently in progress, driven by robust R&D infrastructure and strong patent protection laws, particularly in advanced material and bioengineered coating technologies.
- Europe holds a significant position with approximately 500 patents filed in the past and 180 active filings, focusing on customisable designs and patient-specific innovations.
- Asia-Pacific is rapidly advancing with 450 historical patents and 200 ongoing patents, fueled by expanding healthcare industries and increasing investments in innovative stent graft technologies.
Patent Profile of Key Companies
Several key companies driving innovation and securing intellectual property shape the patent landscape for aortic stent graft. Here is an overview of their patent activities.Cook Medical Technologies LLC
Cook Medical Technologies LLC leads the aortic stent graft patent landscape with over 300 patents filed historically and 120 currently in progress. Their focus on advanced materials and innovative deployment mechanisms drives their patent activity. Future projections suggest over 150 additional patents, reflecting continuous advancements in aortic stent graft technologies.Boston Scientific Scimed Inc
Boston Scientific Scimed Inc. is a key player with 250 patents historically filed and 100 patents currently being pursued. Their commitment to developing advanced deployment systems and customisable designs has propelled their growth. An estimated 130 more patents are expected, showcasing their ongoing innovations and domain influence.Edwards Lifesciences Corp
Edwards Lifesciences Corp holds a prominent position with 200 patents historically filed and 90 patents currently active. Their focus on patient-specific and customisable stent graft designs has driven significant innovation. Future projections indicate over 120 additional patents, reflecting their continuous advancements in this field.Other key players in the industry include Medtronic Vascular Inc., and Advanced Cardiovascular Systems, Inc.
Key Questions Answered in the Global Aortic Stent Graft Patent Landscape Report
- What are the latest technological innovations in aortic stent grafts?
- Which companies are leading the patent filings for aortic stent grafts?
- How many patents have Cook Medical Technologies LLC, Boston Scientific Scimed Inc., and Edwards Lifesciences Corp filed historically and currently?
- What are the major trends in patent filings by product type, specifically for abdominal and thoracic aortic stent grafts?
- How do patent activities differ across end users such as hospitals and ambulatory surgical centers?
- What is the patent landscape for different jurisdictions like the US, Europe, and Asia-Pacific?
- Why does the United States lead in patent filings for aortic stent grafts?
- What are Europe’s contributions to the patent landscape for aortic stent grafts?
- How is the Asia-Pacific region advancing in patent filings for these devices?
- What are the emerging opportunities from the patent portfolios of key companies?
- How do patent strategies impact competitive advantage?
- What are the implications of patent filings in aortic stent graft technology?
- What are the challenges and opportunities in the aortic stent graft patent landscape?
- What are the regulatory and legal considerations?
Reasons to Purchase this Report
This report offers an in-depth analysis of the patent landscape, covering key trends, technological advancements, and regional insights. It provides detailed segmentation and highlights areas of significant innovation and activity. By examining leading companies' strategies and patent portfolios, the report elucidates competitive dynamics and emerging opportunities. Stakeholders will gain valuable information for strategic decision-making, ensuring they stay ahead in the evolving industry. This comprehensive coverage makes it an essential resource for understanding the industry's future direction.This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Cook Medical Technologies LLC
- Boston Scient Scimed Inc
- Edwards Lifesciences Corp
- Medtronic Vascular INC
- Advanced Cardiovascular System
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2024 |
| Forecast Period | 2024 - 2032 |
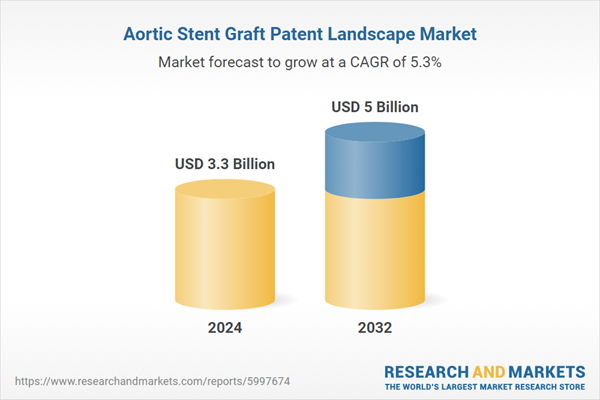
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 5 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |