Patent Landscape Report Coverage
The therapeutic mRNA patent landscape report delves into the dynamic and rapidly evolving patent landscape of therapeutic mRNA. It highlights key innovations in lipid nanoparticles and polymer-based delivery systems, chemical modifications for mRNA stability, and mRNA-based cancer immunotherapies. The report provides a comprehensive analysis of patent filings segmented by design, route of administration, and indication, showcasing leading regions such as the United States, Europe, and Asia. It also profiles major players like Pfizer, Novartis, and Genentech, detailing their patent activities and contributions to advancing mRNA therapies.Global Therapeutic mRNA Patent Outlook
- The therapeutic mRNA market is driven by the rising incidence of chronic diseases and technological advancements in mRNA delivery and stability. Innovations in lipid nanoparticles and polymer-based systems enhance mRNA therapy efficacy, broadening its applications in personalised medicine and vaccines.
- Numerous biotech firms and academic institutions are actively filing patents in the therapeutic mRNA landscape. Pfizer, Novartis, and Genentech are leading the charge with extensive patents on mRNA stability, delivery methods, and therapeutic applications. Their contributions are pivotal in advancing mRNA therapies for various diseases, including cancer and infectious diseases.
- The US leads the patent landscape for therapeutic mRNA, with Germany, the UK, and France focusing on enhancing mRNA modifications and immunogenicity. The Asia Pacific region, particularly China and Japan, is emerging as a key player with numerous patents on cost-effective production methods and novel therapeutic applications. These regions are poised to dominate the patent landscape in the future due to advancements in delivery systems, stability enhancements, and therapeutic efficacy.
Therapeutic mRNA Introduction
Therapeutic mRNA represents a revolutionary approach in modern medicine, using messenger RNA to instruct cells to produce specific proteins that can treat or prevent diseases. Unlike traditional gene therapy, mRNA therapies do not alter the patient’s DNA, offering a safer alternative. These therapies have shown immense potential in various fields, including vaccines, cancer treatment, and rare genetic disorders. The rapid development and success of mRNA COVID-19 vaccines have accelerated research and investment in this area. As technology advances, therapeutic mRNA is poised to transform the landscape of personalised medicine, providing targeted, efficient, and adaptable treatment options.Patents on novel lipid nanoparticles and polymer-based delivery systems are driving the therapeutic mRNA market. These innovations enhance mRNA stability and cellular uptake, improving the efficiency of mRNA therapies. Continuous patent activity in this area reflects ongoing efforts to overcome delivery challenges, expanding the therapeutic potential of mRNA.
- Increasing patents focusing on chemical modifications to improve mRNA stability are shaping the market. These patents cover techniques such as pseudouridine incorporation and 5-methylcytosine modifications. Such advancements ensure sustained protein expression, enhancing the efficacy of mRNA therapies and reflecting significant patent activity aimed at improving therapeutic outcomes.
- Patents on mRNA-based cancer immunotherapies are expanding the landscape. These innovations focus on using mRNA to produce tumour antigens that stimulate the immune system. The surge in patent filings in this area highlights the growing interest in mRNA as a versatile tool for personalized cancer treatment.
Global Therapeutic mRNA Patent Segmentation Analysis
The report provides an in-depth analysis of the patents in this field by the following segmentation :
Analysis by Design
- Self-Amplifying mRNA
- Conventional non-Amplifying mRNA
Analysis by Route of Administration
- Intravenous
- Intramuscular
- Others
Analysis by Indication
- Oncology
- Infectious Diseases
- Autoimmune Diseases
- Others
Therapeutic mRNA Patent Jurisdiction Analysis
The global patent landscape for therapeutic mRNA is rapidly evolving, with significant activity in the United States, Europe, and Asia. Each region reveals unique trends in patent filings and corporate involvement, reflecting regional priorities and advancements in this innovative technology.- The United States leads the patent landscape for therapeutic mRNA with over 700 patents filed. This leadership is driven by substantial investment in biotechnology and a supportive regulatory framework. Key patents focus on advanced delivery systems and mRNA stability, reflecting the country's commitment to pioneering mRNA-based therapies.
- Europe holds approximately 600 patents in the therapeutic mRNA sector, with significant contributions from Germany, the UK, and France. European patents often emphasise innovations in mRNA modifications and immunogenicity enhancements. Collaborative research initiatives and strong funding support these advancements, highlighting Europe’s role in advancing mRNA technology for medical applications.
- Asia, particularly China and Japan, has around 500 patents related to therapeutic mRNA. Rapid technological advancements and robust government support for biotech research drive significant patent activity in this region. Asian patents typically focus on cost-effective production methods and novel therapeutic applications, showcasing the region's growing influence in the global mRNA landscape.
Patent Profile of Key Companies
The patent landscape for therapeutic mRNA is shaped by several key companies driving innovation and securing intellectual property. Here is an overview of their patent activities.Pfizer, Inc.:
Pfizer, Inc. holds a leading position in the therapeutic mRNA patent landscape, with numerous patents focused on vaccine development and delivery systems. Their innovations drive advancements in mRNA stability and immune response optimisation.Novartis AG:
Novartis AG has a robust portfolio in therapeutic mRNA, emphasising cancer immunotherapy and personalized medicine. Their patents focus on innovative mRNA delivery methods and therapeutic applications, enhancing treatment precision and efficacy.Genentech Inc.:
Genentech Inc. is a key player in the therapeutic mRNA patent landscape, with patents centred on advanced mRNA formulations and delivery technologies. Their contributions are pivotal in developing targeted mRNA therapies for various diseases, including oncology and genetic disorders.Other key players in the industry include Amgen Inc., Regeneron Pharma.
Key Questions Answered in the Global Therapeutic mRNA Patent Landscape Report
- What are the implications of patent filings in therapeutic mRNA?
- What are the challenges and opportunities in the therapeutic mRNA patent landscape?
- What are the regulatory and legal considerations?
- What are the major global trends in therapeutic mRNA patents?
- Which regions are leading in therapeutic mRNA patent filings?
- What drives the therapeutic mRNA patent landscape?
- How do self-amplifying mRNA patents differ from conventional ones?
- What advancements are notable in mRNA administration routes?
- Which diseases are most targeted in mRNA patents?
- What are the key patent activities of major companies?
- How do patent trends vary by region?
- What chemical modifications are significant for mRNA stability?
- How is the therapeutic mRNA patent landscape expected to change?
- What is the technological focus of patents in the therapeutic mRNA industry?
Reasons to Purchase this Report
This report provides a comprehensive analysis of the global therapeutic mRNA patent landscape, covering market size, growth trends, and technological advancements. It offers detailed insights into patent segmentation by type and application, highlighting key areas of innovation and activity. By examining the strategies and patent portfolios of leading companies, the report elucidates competitive dynamics and emerging opportunities. Stakeholders will gain valuable information on patent trends, regional developments, and technological breakthroughs, aiding strategic decision-making and fostering advancements in therapeutic mRNA.This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Pfizer, Inc.
- Novartis AG
- Genentech Inc.
- Amgen Inc.
- Regeneron Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2024 |
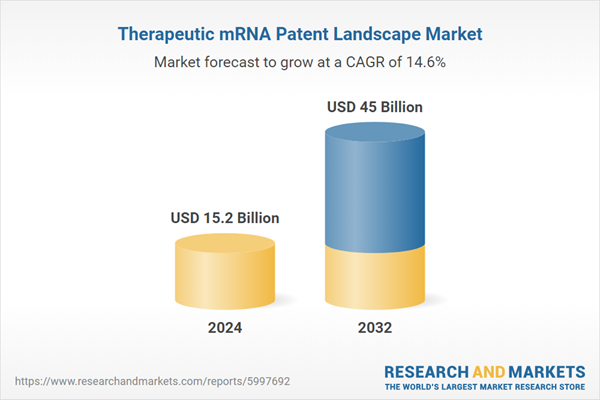
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 15.2 Billion |
| Forecasted Market Value ( USD | $ 45 Billion |
| Compound Annual Growth Rate | 14.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |