Patent Landscape Report Coverage
The report provides a detailed analysis of the mRNA cancer therapeutics patent landscape, focusing on key elements influencing landscape dynamics. It covers jurisdictional trends, with insights into patent activity in the United States, Europe, and Asia. The report also highlights major companies shaping the field, such as Pfizer, Moderna, and Novartis, detailing their contributions and patent portfolios. Additionally, it examines critical drivers of innovation, including advancements in mRNA stability, delivery systems, and immunogenicity. By exploring these factors, the report offers a comprehensive overview of technological progress and competitive developments in mRNA cancer therapeutics.mRNA Cancer Therapeutics Patent Outlook
- The US has over 350 mRNA cancer therapeutics patents due to innovation and regulations. Europe has around 250 patents from collaborations and investments. Asia, mainly China and Japan, has approximately 150 patents due to progress and support.
- Key players in the patent landscape include Pfizer, Inc., with over 200 patents focusing on mRNA stability and delivery systems; Moderna, Inc., holding around 300 patents for lipid nanoparticle delivery and mRNA optimisation; and Novartis AG, with approximately 150 patents on adjuvants and scalable production techniques, driving innovation in mRNA cancer therapeutics.
- Advancements in mRNA stability, delivery systems using lipid nanoparticles and polymer-based carriers, and RNA sequence modifications with immunostimulatory adjuvants enhance immune responses against cancer cells.
mRNA Cancer Therapeutics Introduction
mRNA cancer therapeutics represent a groundbreaking approach in oncology, using messenger RNA to instruct cells to produce proteins that target and destroy cancer cells. This innovative method offers precise, personalised treatments with fewer side effects compared to traditional therapies. By leveraging the body's own cellular machinery, mRNA therapeutics can elicit strong immune responses, providing robust and adaptable cancer treatment options that hold significant promise for improving patient outcomes and advancing cancer care.- Patent activity in mRNA cancer therapeutics is significantly driven by innovations in enhancing mRNA stability. Patents focus on chemical modifications, such as incorporating pseudouridine and 5-methylcytosine, to improve the structural integrity and longevity of mRNA. These modifications help prevent degradation and ensure sustained protein expression in cancer cells, which is crucial for maintaining therapeutic efficacy and reducing the frequency of dosing, thereby directly impacting the patent landscape.
- The development of advanced delivery systems is a major driver of patent activity in mRNA cancer therapeutics. Patents in this domain focus on lipid nanoparticles (LNPs), liposomes, and polymer-based carriers to protect mRNA and facilitate efficient cellular delivery. Innovations include targeting ligands and PEGylation to enhance delivery efficiency, minimise off-target effects, and improve the bioavailability of mRNA therapeutics. These advancements ensure precise delivery to cancer cells, boosting therapeutic effectiveness and expanding the patent landscape.
- Efforts to enhance immunogenicity are pivotal in driving patent activity in mRNA cancer therapeutics. Patents cover RNA sequence modifications, such as optimising codon usage and incorporating immunostimulatory adjuvants, to elicit stronger immune responses against cancer cells. These innovations focus on increasing protein expression and antigen presentation, improving the vaccine's efficacy. Such advancements broaden the application of mRNA cancer treatments, making them more effective and personalised, thereby contributing significantly to the expanding patent landscape.
MRNA Cancer Therapeutics Patent Segmentation Analysis
The report provides an in-depth analysis of the patents in this field by the following segmentation.Analysis by Type
- Self-Amplifying mRNA Vaccines
- Non-Amplifying mRNA Vaccines
Analysis by Route of Administration
- Intravenous
- Intramuscular
- Others
Analysis by Therapeutic Area
- Infectious Diseases
- Oncology
- Others
mRNA Cancer Therapeutics Patent Jurisdiction Analysis
The mRNA cancer therapeutics patent landscape shows significant activity across key jurisdictions. The United States leads with over 350 patents, driven by a strong innovation ecosystem and supportive regulatory framework. Europe follows with around 250 patents, benefitting from robust collaborative networks and significant biotech investments. Asia, particularly China and Japan, accounts for approximately 150 patents, reflecting rapid advancements and substantial government support. Each region faces challenges in harmonising patent laws and navigating regulatory complexities, but ongoing efforts in international collaboration and standardisation are expected to streamline processes, fostering further innovation and growth in mRNA cancer therapeutics globally.Patent Profile of Key Companies
The patent landscape for mRNA cancer therapeutics is shaped by several key companies driving innovation and securing intellectual property. Here is an overview of their patent activities.Pfizer, Inc.
Pfizer, Inc. is a key player in the mRNA cancer therapeutics patent landscape, holding over 200 patents. Their focus on innovative mRNA technologies has led to significant advancements in stabilising mRNA and enhancing delivery systems. Pfizer's strong research capabilities and strategic partnerships bolster their patent portfolio, driving forward the development of effective cancer treatments.
Modernatx Inc.
Modernatx Inc. stands at the forefront of mRNA cancer therapeutics, with a robust patent portfolio of over 300 patents. Known for their pioneering work in mRNA technology, Moderna's patents cover critical areas such as lipid nanoparticle delivery systems and mRNA sequence optimisation. Their continuous innovation and extensive clinical trials underpin their leading position in this domain.
Novartis AG
Novartis AG has established a significant presence in the mRNA cancer therapeutics patent landscape, with around 150 patents. Their patents focus on novel adjuvants and scalable production techniques, aiming to improve the efficacy and accessibility of mRNA-based cancer treatments. Novartis' investment in R&D and collaborations with research institutions drive their contributions to this field.Other key players in the industry include Genentech Inc., Immatics Biotechnologies GmbH.
Key Questions Answered in the MRNA Cancer Therapeutics Patent Landscape Report
- What is the current industry size for mRNA cancer therapeutics?
- What is the expected growth rate of the mRNA cancer therapeutics industry?
- Which regions are leading in mRNA cancer therapeutics patents?
- Which companies are most active in mRNA cancer therapeutics patents?
- What are the key drivers of patent activity in mRNA cancer therapeutics?
- How do self-amplifying and non-amplifying mRNA vaccines differ in patents?
- What are the common routes of administration for mRNA cancer therapeutics?
- Which therapeutic areas are most covered by mRNA cancer therapeutics patents?
- What challenges exist in the patenting of mRNA cancer therapeutics?
- How do innovations in delivery systems affect mRNA cancer therapeutics patents?
- How do patent strategies impact competitive advantage?
- What are the implications of patent filings in mRNA cancer therapeutics?
- What are the challenges and opportunities in the mRNA cancer therapeutics patent landscape?
- What are the regulatory and legal considerations?
- What technological innovations have recently emerged in mRNA cancer therapeutics?
Reasons to Purchase this Report
This report provides a comprehensive analysis of the mRNA cancer therapeutics patent landscape, essential for understanding the current state and future trends of this innovative field. By examining patent activity across various segments, including type, route of administration, and therapeutic areas, the report highlights key drivers of growth and technological advancements. It offers valuable insights into the competitive landscape, key players, and jurisdictional trends. This information is crucial for stakeholders seeking to navigate the complexities of the mRNA industry, make informed strategic decisions, and identify opportunities for investment and collaboration in this rapidly evolving sector.This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Pfizer, Inc.
- Novartis AG
- Genentech Inc.
- Immatics Biotechnologies GmbH
- Modernatx Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2024 |
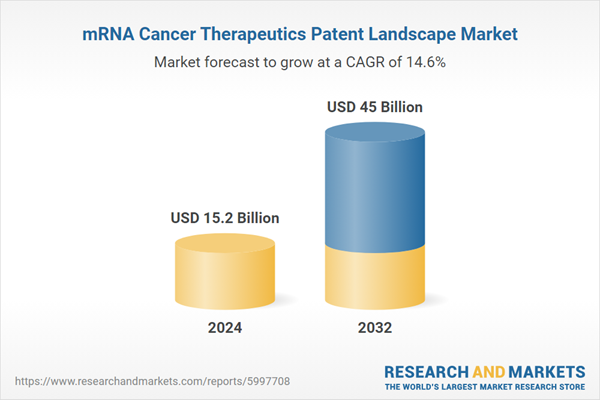
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 15.2 Billion |
| Forecasted Market Value ( USD | $ 45 Billion |
| Compound Annual Growth Rate | 14.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |