Chronic Inflammatory Demyelinating Polyneuropathy Therapeutics Market Report and Forecast 2024-2032
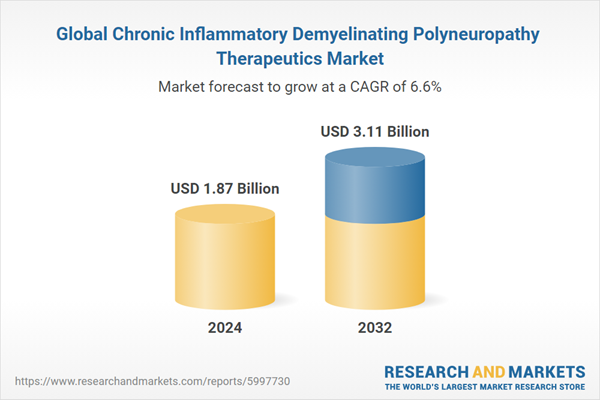
The chronic inflammatory demyelinating polyneuropathy therapeutics market was valued at USD 1.75 billion in 2023. It is expected to grow at a CAGR of 6.60% during the forecast period of 2024-2032 and attain a value of USD 3.11 billion by 2032. It is driven by the rising introduction of new therapeutic options and increased R&D activities focused on managing the disorder across the 8 major markets.Global Chronic Inflammatory Demyelinating Polyneuropathy Therapeutics Market Analysis
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is a rare autoimmune disorder characterised by progressive weakness and impaired sensory function in the legs and arms. CIDP is caused by damage to the myelin sheath, the protective covering of the nerves, leading to deteriorating nerve function. The global market for CIDP therapeutics encompasses various treatment modalities, including intravenous immunoglobulin (IVIG), corticosteroids, and plasma exchange, aimed at managing symptoms and improving the quality of life for patients. The market has witnessed significant growth due to advancements in medical research and increased awareness of rare neurological disorders.Market Drivers
- Rising Prevalence of CIDP: An increase in the incidence and diagnosis of CIDP globally has spurred demand for effective therapeutics. Improved diagnostic techniques and heightened awareness among healthcare professionals contribute to earlier detection and treatment initiation.
- Advancements in Treatment Modalities: Continuous research and development activities have led to innovative therapies, including novel immunotherapies and biologics. These advancements offer more effective and targeted treatment options, driving market growth.
- Government and Non-Governmental Support: Various governmental bodies and non-profit organisations are investing in research and development for rare diseases, including CIDP. Funding and grants have accelerated the development of new therapies, fostering market expansion.
- Favourable Reimbursement Policies: In several regions, insurance companies and healthcare systems provide coverage for CIDP treatments, making them more accessible to patients. This support reduces the financial burden on patients and encourages the uptake of therapeutic interventions.
Market Challenges
- High Cost of Treatments: The cost associated with CIDP treatments, particularly IVIG and biologics, is substantial. This high cost can be prohibitive for patients without adequate insurance coverage, limiting market growth.
- Limited Awareness and Diagnosis in Developing Regions: In low and middle-income countries, limited awareness and diagnostic capabilities for CIDP pose significant challenges. Lack of specialised healthcare infrastructure and trained professionals can result in underdiagnosis and undertreatment.
- Adverse Effects of Long-Term Therapies: Chronic use of immunosuppressive therapies and corticosteroids can lead to adverse effects, including increased susceptibility to infections and other complications. These side effects can impact patient compliance and the long-term efficacy of treatments.
- Regulatory Hurdles: The stringent regulatory requirements for the approval of new therapies can delay market entry. Ensuring safety and efficacy through rigorous clinical trials is essential but time-consuming and costly.
Future Opportunities
- Development of Novel Therapies: Ongoing research in immunology and neurology is expected to yield new therapeutic options for CIDP. Innovations in gene therapy, monoclonal antibodies, and other biologics present significant opportunities for improved treatments.
- Expansion in Emerging Markets: Increasing healthcare investment and improving diagnostic infrastructure in emerging markets offer substantial growth potential. Raising awareness and training healthcare professionals in these regions can enhance the diagnosis and management of CIDP.
- Personalised Medicine: Advances in personalised medicine and biomarkers can lead to more tailored treatment approaches, improving patient outcomes. Customised therapies based on individual patient profiles can enhance efficacy and reduce adverse effects.
- Collaborative Research and Development: Collaboration between pharmaceutical companies, academic institutions, and research organisations can expedite the development of new therapies. Public-private partnerships and international collaborations can pool resources and expertise, driving innovation in CIDP therapeutics.
Global Chronic Inflammatory Demyelinating Polyneuropathy Therapeutics Market Trends
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is a rare, progressive autoimmune disorder that affects the peripheral nerves, leading to muscle weakness, impaired motor function, and sensory disturbances. The global market for CIDP therapeutics involves various treatment approaches such as intravenous immunoglobulin (IVIG), corticosteroids, plasma exchange, and emerging biologic therapies. This market is driven by advancements in medical research, increasing awareness of the disease, and the development of novel therapeutic options.Market Trends
- Rise in Biologics and Monoclonal Antibodies: The CIDP therapeutics market is witnessing a significant shift towards biologics and monoclonal antibodies. These advanced treatments offer targeted action against the immune pathways involved in CIDP, providing better efficacy and safety profiles compared to traditional therapies. The development of drugs like rituximab and eculizumab is paving the way for a new era in CIDP management.
- Increasing Adoption of Subcutaneous Immunoglobulin (SCIG): Subcutaneous immunoglobulin (SCIG) is gaining popularity as an alternative to intravenous immunoglobulin (IVIG). SCIG offers the advantages of home administration, reduced infusion-related side effects, and improved patient compliance. The convenience and effectiveness of SCIG are driving its adoption, particularly in regions with advanced healthcare infrastructure.
- Focus on Early Diagnosis and Intervention: There is a growing emphasis on the early diagnosis and intervention of CIDP. Advances in diagnostic tools, such as nerve conduction studies and magnetic resonance imaging (MRI), enable earlier detection and treatment initiation, which can significantly improve patient outcomes. Early intervention strategies are becoming a key focus for healthcare providers and researchers.
- Patient-Centric Treatment Approaches: The trend towards personalised medicine is influencing the CIDP therapeutics market. Tailoring treatment plans based on individual patient profiles, including genetic, environmental, and lifestyle factors, is enhancing the effectiveness of therapies. Personalised treatment approaches are leading to better management of the disease and improved quality of life for patients.
- Expansion of Clinical Trials and Research: Increased investment in clinical trials and research is a notable trend in the CIDP therapeutics market. Pharmaceutical companies and research institutions are collaborating to explore new therapeutic targets and develop innovative treatments. The expansion of clinical trials is accelerating the approval process for new drugs, contributing to market growth.
- Growing Awareness and Patient Advocacy: Patient advocacy groups and non-profit organisations are playing a crucial role in raising awareness about CIDP. Educational campaigns, support groups, and awareness programmes are helping to disseminate information about the disease, its symptoms, and available treatments. Increased awareness is driving early diagnosis and encouraging patients to seek appropriate medical care.
- Regional Market Expansion: The CIDP therapeutics market is expanding in emerging economies due to improving healthcare infrastructure and increasing healthcare expenditure. Countries in Asia-Pacific and Latin America are witnessing a rise in the availability of CIDP treatments. Market players are focusing on these regions to tap into the growing patient population and unmet medical needs.
- Technological Advancements in Drug Delivery Systems: Technological innovations in drug delivery systems are enhancing the efficacy and convenience of CIDP treatments. Advances such as sustained-release formulations, novel delivery devices, and wearable injectors are improving patient adherence to therapy and optimising treatment outcomes.
- Integration of Telemedicine and Digital Health Solutions: The integration of telemedicine and digital health solutions is transforming the management of CIDP. Remote monitoring, teleconsultations, and digital health platforms are facilitating continuous patient care, reducing the need for frequent hospital visits, and enabling timely interventions. The adoption of these technologies is improving patient engagement and treatment adherence.
- Regulatory Support and Orphan Drug Designation: Regulatory authorities are providing support for the development of CIDP treatments through orphan drug designation and expedited approval processes. This regulatory encouragement is fostering innovation and accelerating the availability of new therapies in the market.
Global Chronic Inflammatory Demyelinating Polyneuropathy Therapeutics Market Segmentation
Market Breakup by Drug Type
- Corticosteroids
- Intravenous immunoglobulin therapy (IVIG)
- Plasmapheresis (Plasma Exchange)
- Immunotherapy
- Stem cell transplant
- Others
Market Breakup by Route of Administration
- Oral
- Injectable
- Others
Market Breakup by Gender
- Male
- Female
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Home Healthcare
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Global Chronic Inflammatory Demyelinating Polyneuropathy Therapeutics Market Competitive Landscape
The competitive landscape of the global CIDP therapeutics market includes key players such as CSL Behring, ADMA Biologics, Inc., Pfizer Inc., UCB S.A, Bio Products Laboratory Ltd., Grifols, Teva Pharmaceutical Industries Ltd., and Sun Pharmaceutical Industries Ltd. Common market activities among these companies include mergers and acquisitions to expand their portfolios and market reach. They engage in extensive research initiatives to develop innovative therapies and improve existing treatments. Product introductions are frequent, with companies launching new immunoglobulins, corticosteroids, and biologics. Strategic partnerships and collaborations are also prevalent, aimed at enhancing research capabilities and accelerating the development and distribution of CIDP therapeutics. These activities collectively drive market growth and foster competitive dynamics within the industry.Key Questions Answered in the Report
- What is the current and future performance of the chronic inflammatory demyelinating polyneuropathy therapeutics market?
- What are the main challenges facing the chronic inflammatory demyelinating polyneuropathy therapeutics market?
- What are the key drivers of the chronic inflammatory demyelinating polyneuropathy therapeutics market?
- What emerging trends are shaping the future of the chronic inflammatory demyelinating polyneuropathy therapeutics market?
- Why do hospitals dominate the CIDP therapeutics market over other end user segments?
- Why do hospital pharmacies lead the CIDP therapeutics market over other distribution channels?
- Why do EU-4 countries and the United Kingdom hold substantial shares in the CIDP therapeutics market?
- How do research initiatives and frequent product introductions impact the CIDP therapeutics market?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the chronic inflammatory demyelinating polyneuropathy therapeutics market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the chronic inflammatory demyelinating polyneuropathy therapeutics market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the chronic inflammatory demyelinating polyneuropathy therapeutics industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- CSL Behring
- ADMA Biologics, Inc.
- Pfizer Inc.
- UCB S.A
- Bio Products Laboratory Ltd.
- Grifols
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 1.87 Billion |
| Forecasted Market Value ( USD | $ 3.11 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |