Global Infectious Disease Molecular Diagnostics Market Overview
Infectious disease molecular diagnostic devices include specialized instruments or platforms designed for the detection and identification of infectious agents, such as viruses, bacteria, fungi, and parasites, through molecular methods. These devices employ various molecular diagnostic technologies to amplify and analyze nucleic acids (e.g., DNA or RNA) specific to pathogens present in clinical samples obtained from patients.The global burden of infectious diseases continues to increase due to factors such as population growth and antimicrobial resistance. As a result, there is a growing need for accurate and rapid diagnostic tests to identify pathogens responsible for infection. Moreover, governments, healthcare systems, public health agencies are investing in molecular diagnostics to enhance their capacity for early detection, surveillance, and containment of outbreaks.
There is a growing demand for point-of-care (POCT) molecular diagnostic tests that can deliver rapid results at the bedside or in community settings. POCT solutions enable timely diagnosis, treatment initiation, and containment of infectious diseases, particularly in resource-limited areas which is expected to create great opportunities for the growth of the market.
Global Infectious Disease Molecular Diagnostics Market Growth Drivers
Rising Demand for Diagnostics Solution
As the incidence of infectious diseases increases, there is a corresponding rise in the demand for diagnostic solutions to identify pathogens causing these diseases. Molecular diagnostics offer high sensitivity and specificity, enabling healthcare providers to detect infectious agents with greater accuracy than traditional methods, such as culture-based techniques.In the face of outbreaks or epidemics, early detection and surveillance are crucial for controlling the spread of infectious diseases. Molecular diagnostics facilitate early identification of pathogens, allowing for prompt intervention measures. The urgency to contain outbreaks drives the adoption of molecular diagnostic tests, thereby boosting market value.
Surge in Infectious Diseases
According to the World Health Organization's (WHO) annual estimates, there are globally 300-500 million cases of malaria, 333 million cases of sexually transmitted diseases (syphilis, gonorrhea, chlamydia, and trichomonas), 33 million cases of HIV/AIDS, 14 million people infected with tuberculosis, and 3-5 million cases of cholera. The increase in infectious diseases driving demand for diagnostic solutions, prompting technological advancements, expanding testing capabilities, attracting funding, and raising public awareness about the importance of early detection and surveillance.Increasing Technological Innovations Expected to Propel Infectious Disease Molecular Diagnostics Market Demand
Technological advancements play a pivotal role in driving growth in the market of infectious disease molecular diagnostics by enhancing the performance, accessibility, and versatility of diagnostic tests. Techniques such as real-time PCR, digital PCR, and next-generation sequencing (NGS) enable the detection of low levels of pathogens in clinical samples, improving diagnostic accuracy and reducing false-negative results. Multiplex assays allow the simultaneous detection of multiple pathogens or genetic targets in a single test, offering significant time and cost savings compared to traditional single-target assays.
Hence, the technological advancements continue to shape the landscape of infectious disease molecular diagnostics, driving innovation, improving performance, and expanding the market growth.
Global Infectious Disease Molecular Diagnostics Market Trends
The market is witnessing several trends and developments to improve the current global scenario. Some of the notable trends are as follows:Expansion of Point-of-Care Testing
There's a growing trend towards the adoption of point of care molecular diagnostics, driven by the need for rapid and decentralized testing. Advancements such as portable molecular diagnostic devices are being developed to enable on-site testing in various settings, including clinics, emergency departments, and remote areas with limited access to laboratory facilities, contributing to market growth. For instance, several RT-PCR systems that use micro fluidic technology including Cepheid GeneXpert have been granted the clinical laboratory improvement amendment (CLIA) waiver by the United States Food and Drug administration (FDA), which allowing their use in POC settings.Integration of Next Generation Sequencing
Next-generation sequencing (NGS) technologies are increasingly being integrated into infectious disease molecular diagnostics workflows, enabling comprehensive genomic analysis of pathogens. NGS offers insights into pathogen evolution, antimicrobial resistance, and outbreak investigation, driving its adoption in clinical and public health settings. The increasing technological advancements are augmenting the growth of the market.Rise in Strategic Partnerships
The infectious disease molecular diagnostics market value is influenced by strategic mergers and acquisitions activities among manufacturers, healthcare providers, and technology companies that are driving innovation and fostering market expansion. These collaborations are accelerating the development of advanced products and augmenting the market reach globally. For instance, in April 2023, ALPCO-GeneProof and Thermo F isher announced a strategic partnership intended to bring TaqPath Menu-GeneProof PCR kits to the market. The partnership combined the strengths of ALPCO-GeneProof's expertise in molecular diagnostics with Thermo Fisher's robust supply chain and support systems.Global Infectious Disease Molecular Diagnostics Market Segmentation
The report titled “Infectious Disease Molecular Diagnostics Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Instruments
- Benchtop
- Portable
- Reagents
- Services
Market Breakup by Technology
- Polymerase Chain Reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
Market Breakup by Application
- Respiratory Diseases
- Gastrointestinal Tract Infections
- Sexually Transmitted Infections
- Drug Resistance Diseases
- Others
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Diagnostic Laboratories
- Research Institutes
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Infectious Disease Molecular Diagnostics Market Share
Market Segmentation Based on Technology is Anticipated to Witness Substantial Growth
By technology, the market is segmented into polymerase chain reaction (PCR), in situ hybridization, isothermal nucleic acid amplification technology (INAAT), chips and microarrays, mass spectrometry, sequencing, transcription mediated amplification and others. The polymerase chain reaction (PCR) segment is dominating the market as it is one of the most widely used and established technologies in infectious disease molecular diagnostics. It allows for the amplification and detection of specific DNA or RNA sequences of pathogens with high sensitivity and specificity. Real-time PCR enables quantitative measurement of target nucleic acids and is commonly used for viral load monitoring and detection of bacterial, viral, and fungal infections.Global Infectious Disease Molecular Diagnostics Market Analysis by Region
Based on region, the market covers North America, Europe, Asia Pacific, Latin America, as well as Middle East and Africa. North America is dominating the market share due to increasing technological innovation. North America, particularly United States is a leading hub for innovation and research in molecular diagnostics. Moreover, the government agencies, and private investors in North America allocate significant funding and resources to support research and development in infectious disease diagnostics market.Europe also holds a significant infectious disease molecular diagnostics market value due to region's robust healthcare infrastructure supports the adoption and integration of molecular diagnostic technologies into routine clinical practice, facilitating widespread testing for infectious diseases across various healthcare settings. Additionally, Asia Pacific is expected to witness substantial market growth fueled by the growing patient pool suffering from infectious diseases and increasing collaboration and funding initiatives.
Leading Players in the Global Infectious Disease Molecular Diagnostics Market
The key features of the market report include patent analysis and strategic initiatives including partnerships, and collaborations analysis by the leading players. The major companies in the market are as follows:Becton, Dickinson and Company
BD is a leading global medical technology company that offers a wide range of products and solutions, including infectious disease molecular diagnostic devices. BD has a significant presence in the infectious disease molecular diagnostics market, offering innovative solutions to healthcare providers, laboratories, and public health agencies worldwide.BioMérieux SA
BioMérieux is a prominent global leader in in-vitro diagnostics, including molecular diagnostics for infectious diseases. The company offers a range of innovative molecular diagnostic devices designed to detect and identify pathogens with accuracy and efficiency.QIAGEN
QIAGEN is a leading global provider of molecular diagnostics solutions, offering a range of innovative products and platforms for the detection and identification of infectious diseases.Other key players in the market include F. Hoffmann-La Roche Ltd., Abbott, Agilent Technologies, Inc., Danaher Corporation, Hologic, Inc., Sysmex Corporation, and Illumina, Inc. among others.
Key Questions Answered in the Global Infectious Disease Molecular Diagnostics Market Report
- What was the global infectious disease molecular diagnostics market value in 2024?
- What is the global infectious disease molecular diagnostics market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on product?
- What is market segmentation based on technology?
- What is market segmentation based on application?
- What are the major factors aiding the global infectious disease molecular diagnostics market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major market trends influencing the market?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- How does the prevalence of infectious diseases affect the market landscape?
- How does the growing technological advancements impact the market size?
- How do the increasing trend of POC testing affect the market landscape?
- Which application area is expected to have a high market value in the coming years?
- Who are the key players involved in the infectious disease molecular diagnostics market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Becton, Dickinson and Company
- bioMérieux SA
- QIAGEN
- F. Hoffmann-La Roche Ltd
- Abbott
- Danaher Corporation
- Agilent Technologies, Inc.
- Hologic, Inc.
- Sysmex Corporation
- Illumina, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
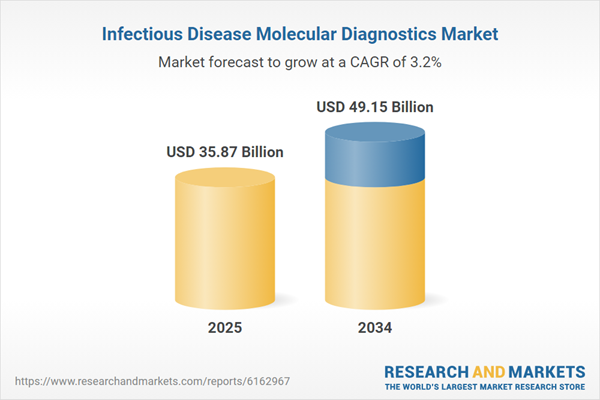
| Estimated Market Value ( USD | $ 35.87 Billion |
| Forecasted Market Value ( USD | $ 49.15 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |