Global Left Atrial Appendage (LAA) Closure Devices Market - Key Trends and Drivers Summarized
What Are Left Atrial Appendage (LAA) Closure Devices and Why Are They Critical?
Left Atrial Appendage (LAA) closure devices are specialized medical tools designed to reduce the risk of stroke in patients with atrial fibrillation (AF), a common heart rhythm disorder. The LAA is a small, ear-shaped pouch in the left atrium of the heart where blood clots are likely to form in patients with AF, due to irregular blood flow. If these clots travel to the brain, they can cause a stroke. Traditionally, stroke prevention in AF patients has relied on anticoagulant medications to reduce blood clot formation. However, not all patients can tolerate these drugs due to the risk of bleeding complications. LAA closure devices offer an alternative by physically sealing off the LAA, thereby preventing clots from entering the bloodstream and causing a stroke. This intervention is especially critical for patients who are at high risk of stroke but cannot safely take long-term anticoagulants. As such, LAA closure devices are becoming an increasingly vital option in the management of atrial fibrillation, offering a potentially life-saving solution for a growing patient population.How Have Advances in Technology Enhanced LAA Closure Devices?
Technological advancements have significantly improved the design, safety, and efficacy of Left Atrial Appendage closure devices. Modern devices are typically implantable and delivered through minimally invasive catheter-based procedures, which involve threading the device through the blood vessels to the heart, where it is then deployed to seal the LAA. These procedures are guided by advanced imaging technologies, such as transesophageal echocardiography and fluoroscopy, which allow for precise placement of the device. Improvements in device materials and engineering have also led to the development of smaller, more flexible, and more biocompatible devices that conform better to the anatomy of the LAA, reducing the risk of complications such as device embolization or incomplete closure. Additionally, newer-generation devices are designed to be retrievable and repositionable, providing the operator with more control during the procedure and further enhancing patient safety. These innovations have expanded the patient eligibility for LAA closure procedures and have contributed to better clinical outcomes, making these devices a more viable and attractive option for stroke prevention in AF patients.Why Are Healthcare Providers and Patients Turning to LAA Closure Devices?
The growing interest in LAA closure devices among healthcare providers and patients is driven by the increasing awareness of the limitations of long-term anticoagulant therapy and the desire for safer, more effective stroke prevention options. Many patients with atrial fibrillation are either at high risk of bleeding or experience adverse effects from anticoagulant medications, which can significantly impact their quality of life and overall health. LAA closure devices offer a one-time, minimally invasive alternative that can dramatically reduce the risk of stroke without the need for ongoing medication, which is particularly appealing to patients who are concerned about the long-term use of blood thinners. For healthcare providers, these devices provide a critical tool in managing stroke risk in a broader range of patients, particularly those who are contraindicated for anticoagulants. The ability to perform the procedure percutaneously, with relatively quick recovery times and minimal hospital stays, further enhances the appeal of LAA closure devices. As the procedure becomes more widely adopted and as clinical data continue to demonstrate its efficacy and safety, both patients and providers are increasingly considering LAA closure as a preferred strategy for stroke prevention in atrial fibrillation.What Factors Are Driving the Growth of the LAA Closure Devices Market?
The growth in the Left Atrial Appendage closure devices market is driven by several key factors, reflecting broader trends in cardiovascular care and patient management. A primary driver is the increasing prevalence of atrial fibrillation worldwide, particularly among the aging population, which is more susceptible to both AF and stroke. As the number of AF patients rises, so too does the need for effective stroke prevention strategies, thereby fueling demand for LAA closure devices. Advances in device technology, including the development of more effective, safer, and easier-to-use devices, have also spurred market growth by expanding the patient population eligible for these procedures. Additionally, the limitations and risks associated with long-term anticoagulant therapy, such as bleeding complications, have led both patients and physicians to seek alternative stroke prevention methods, further driving the adoption of LAA closure devices. The increasing availability of these procedures in a wider range of healthcare settings, including outpatient facilities, has made them more accessible to patients, contributing to market expansion. Furthermore, growing clinical evidence supporting the safety and efficacy of LAA closure, along with favorable reimbursement policies in many regions, has bolstered both physician confidence and patient acceptance, ensuring continued growth in the market for these devices in the coming years.Report Scope
The report analyzes the Left Atrial Appendage (LAA) Closure Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Device Type (Endocardial LAA Devices, Epicardial LAA Devices); End-Use (Hospitals End-Use, Ambulatory Surgery Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Endocardial LAA Devices segment, which is expected to reach US$4.7 Billion by 2030 with a CAGR of a 16.7%. The Epicardial LAA Devices segment is also set to grow at 13.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $614.8 Million in 2024, and China, forecasted to grow at an impressive 21.6% CAGR to reach $1.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Left Atrial Appendage (LAA) Closure Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Left Atrial Appendage (LAA) Closure Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Left Atrial Appendage (LAA) Closure Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Inc., Acutus Medical, Inc., AtriCure, Inc., Biosense Webster, Inc., Boston Scientific Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 52 companies featured in this Left Atrial Appendage (LAA) Closure Devices market report include:
- Abbott Laboratories, Inc.
- Acutus Medical, Inc.
- AtriCure, Inc.
- Biosense Webster, Inc.
- Boston Scientific Corporation
- Carag AG
- Cardia Inc.
- Johnson & Johnson Services, Inc.
- Lepu Medical Technology (Beijing) Co., Ltd.
- Lifetech Scientific Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories, Inc.
- Acutus Medical, Inc.
- AtriCure, Inc.
- Biosense Webster, Inc.
- Boston Scientific Corporation
- Carag AG
- Cardia Inc.
- Johnson & Johnson Services, Inc.
- Lepu Medical Technology (Beijing) Co., Ltd.
- Lifetech Scientific Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 286 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
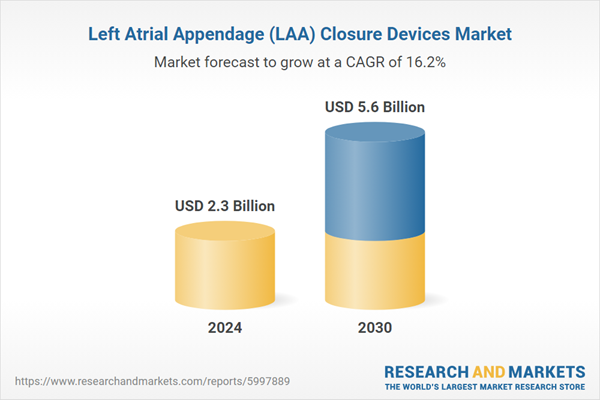
| Estimated Market Value ( USD | $ 2.3 Billion |
| Forecasted Market Value ( USD | $ 5.6 Billion |
| Compound Annual Growth Rate | 16.2% |
| Regions Covered | Global |