Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite challenges such as high drug costs and regulatory obstacles, the market offers significant opportunities for growth and innovation. Major players are actively developing new therapies to address unmet needs, driving further market expansion and advancement in psoriasis treatment in Japan.
Key Market Drivers
Rising Prevalence of Psoriasis
The growing number of individuals diagnosed with psoriasis directly boosts the demand for treatment options. As the prevalence of psoriasis rises, more patients seek effective therapies to manage their condition. The prevalence of self-reported psoriasis (PsO) and/or psoriatic arthritis (PsA) in the Japanese UPLIFT subgroup (2%) was notably higher compared to the estimated prevalence of diagnosed PsO and/or PsA reported in a nationwide study using the Japanese national claims database (0.3%). However, this prevalence was lower than that observed in the overall UPLIFT survey.This increased demand translates into a larger market for psoriasis drugs, as pharmaceutical companies and healthcare providers focus on developing and delivering new and improved treatment options. An increasing prevalence of psoriasis expands the target patient population for psoriasis drugs. This larger patient pool includes individuals with varying degrees of the disease, from mild to severe. Pharmaceutical companies and healthcare providers are motivated to address the needs of this diverse patient population, leading to the development of a wider range of therapies, including biologics, systemic drugs, and topical treatments.
The rising prevalence of psoriasis creates substantial market opportunities for pharmaceutical companies. Companies see potential for growth in both established and emerging markets. This encourages investment in research and development to innovate new treatments and improve existing ones. Additionally, the increased patient base offers opportunities for companies to expand their market share and revenue through both new drug launches and extended indications for existing therapies. As the prevalence of psoriasis rises, there is a heightened focus on drug development and innovation to address the growing patient needs.
The pharmaceutical industry is incentivized to invest in the research and development of novel therapies, including targeted biologics and personalized medicine approaches. This focus on innovation leads to the introduction of more effective and efficient treatments, further driving market growth. The growing number of psoriasis cases contributes to increased healthcare expenditure. Government and private sector spending on psoriasis management, including drug therapies and healthcare services, rises in response to the higher patient burden. This increased expenditure supports market growth by facilitating the availability and affordability of psoriasis drugs and enabling access to advanced treatments.
The rising prevalence of psoriasis raises awareness among healthcare professionals and the general public. Greater awareness leads to improved diagnosis rates, as healthcare providers are more likely to identify and diagnose psoriasis cases earlier. Enhanced diagnostic capabilities contribute to the earlier initiation of treatment, driving demand for psoriasis drugs and promoting market growth. A higher prevalence of psoriasis often leads to increased patient advocacy and support. Patient advocacy groups work to raise awareness, provide education, and influence healthcare policies.
Their efforts help to highlight the need for effective treatments and support the development of new therapies, driving growth in the psoriasis drugs market by ensuring that patient needs are met and that treatments are widely accessible. The rising prevalence of psoriasis can lead to greater government and health insurance support for psoriasis treatments. Governments may implement policies to subsidize the cost of psoriasis drugs or provide funding for research and development. Health insurance providers may expand coverage for psoriasis treatments, making medications more accessible to patients and stimulating market growth.
Advancements in Treatment Technologies
Recent innovations in psoriasis treatment technologies have led to the development of new therapeutic classes that address the limitations of previous treatments. The advent of biologics, such as TNF-alpha inhibitors, IL-17 inhibitors, and IL-23 inhibitors, has revolutionized psoriasis management. These drugs target specific cytokines involved in the inflammatory process, offering more precise and effective treatment options. The availability of these advanced therapies caters to patients with moderate to severe psoriasis and drives market expansion. New oral small molecules, like Janus kinase (JAK) inhibitors, have emerged as viable alternatives to biologics. These medications offer convenience and effectiveness, expanding the range of treatment options available to patients and enhancing market growth.Advancements in drug delivery systems improve the efficacy, convenience, and adherence to psoriasis treatments. Innovations in long-acting injectable formulations reduce the frequency of administration. These formulations enhance patient compliance by minimizing the need for frequent visits to healthcare providers, thereby increasing the adoption of advanced therapies. New topical formulations, such as novel delivery systems for corticosteroids and vitamin D analogs, improve drug penetration and efficacy. These advancements make topical treatments more effective and appealing to patients, contributing to market growth. The development of user-friendly injection devices and auto-injectors simplifies the administration of biologics.
These devices enhance patient convenience and adherence, supporting the growth of the psoriasis drugs market. The shift towards personalized medicine in psoriasis treatment is driving market growth by tailoring therapies to individual patient needs. Advances in genomics and biomarker research enable the identification of specific genetic markers and disease subtypes.
Personalized treatment plans based on these insights allow for more targeted and effective therapies, improving patient outcomes and driving demand for advanced treatments. Personalized medicine approaches allow for the customization of treatment regimens based on patient characteristics, such as disease severity, comorbidities, and previous treatment responses. This tailored approach ensures that patients receive the most appropriate and effective therapies, promoting market growth.
Advancements in diagnostic technologies contribute to the growth of the psoriasis drugs market by enabling early and accurate diagnosis. New imaging technologies, such as high-resolution skin imaging and laser-based methods, provide detailed visualization of psoriasis lesions. Accurate diagnosis and disease monitoring facilitate timely treatment interventions and drive demand for effective psoriasis drugs. The discovery of novel biomarkers associated with psoriasis helps in diagnosing the condition more precisely and predicting treatment responses. Enhanced diagnostic capabilities lead to better patient stratification and management, contributing to market growth.
Digital health technologies are transforming psoriasis management and driving market growth through improved patient engagement and treatment adherence. Mobile health applications allow patients to track their symptoms, medication adherence, and treatment outcomes. These apps provide valuable data to healthcare providers and facilitate personalized treatment adjustments, enhancing the effectiveness of psoriasis therapies and fostering market growth. Telemedicine platforms enable remote consultations and monitoring of psoriasis patients. This technology expands access to care, particularly in rural or underserved areas, and promotes the use of advanced treatments by making them more accessible to a broader patient population.
Ongoing investment in research and development (R&D) drives innovation in psoriasis treatment technologies, contributing to market growth. Increased investment in R&D by pharmaceutical companies leads to the discovery of new treatment modalities and improvements in existing therapies. This investment supports the development of cutting-edge treatments and drives market expansion. Collaboration between academic institutions, research organizations, and the pharmaceutical industry accelerates the development of novel therapies and technologies.
These collaborative efforts foster innovation and contribute to the growth of the psoriasis drugs market. Advancements in treatment technologies drive the growth of the Japan Psoriasis Drugs Market by introducing new therapeutic classes, enhancing drug delivery systems, embracing personalized medicine approaches, improving diagnostic technologies, integrating digital health solutions, and fostering increased R&D investment. These technological advancements enhance the effectiveness, convenience, and accessibility of psoriasis treatments, leading to greater market demand and continued growth.
Increased Healthcare Expenditure and Access
Increased government spending on healthcare significantly impacts the psoriasis drugs market by making advanced treatments more accessible. In 2021, Japan’s national medical care expenses reached approximately 45 trillion Japanese yen, marking an increase from about 39.2 trillion Japanese yen in fiscal year 2012. The Japanese government’s commitment to expanding its healthcare budget directly supports the availability of psoriasis treatments. This includes funding for new drug development, subsidizing the cost of expensive biologics, and supporting research into new therapies. Government subsidies and favorable reimbursement policies reduce the financial burden on patients for psoriasis treatments.By covering a larger portion of treatment costs, these policies enhance patient access to advanced medications and stimulate market growth. Improvements in health insurance coverage contribute to market growth by enhancing patient access to psoriasis treatments. Health insurance providers are increasingly covering newer and more expensive psoriasis drugs, including biologics and advanced topical treatments. This expanded coverage ensures that a larger number of patients can afford and access effective therapies. Expanded insurance coverage reduces out-of-pocket expenses for patients, making it easier for them to adhere to prescribed treatments. Lower financial barriers encourage patients to seek treatment and follow prescribed regimens, contributing to higher market demand.
Enhanced access to specialized dermatological care drives market growth by facilitating timely and accurate treatment of psoriasis. The expansion of healthcare infrastructure and resources results in a greater number of dermatologists and specialized clinics. Improved access to these specialists ensures that more patients receive appropriate and timely psoriasis diagnosis and treatment. The integration of telemedicine services enhances access to specialized care for patients in remote or underserved areas. Telemedicine allows patients to consult with dermatologists and receive prescriptions for advanced psoriasis treatments without needing to travel long distances.
Advancements in healthcare infrastructure contribute to the growth of the psoriasis drugs market by improving the overall quality and accessibility of care. The development and upgrading of healthcare facilities, including hospitals and outpatient clinics, provide better environments for the diagnosis and treatment of psoriasis. State-of-the-art facilities support the use of advanced treatment technologies and enhance patient care. Increased investment in health technology, such as electronic health records (EHR) systems and diagnostic tools, improves the efficiency and effectiveness of psoriasis management. Better health technology supports accurate diagnosis and personalized treatment plans, driving market growth.
Increased healthcare expenditure often leads to higher levels of patient awareness and engagement, which drives market growth. Government and healthcare organizations conduct public health campaigns to raise awareness about psoriasis and available treatment options. These campaigns educate patients about the importance of seeking treatment and adhering to prescribed therapies, increasing demand for psoriasis drugs. Enhanced healthcare spending supports patient education and support programs that help individuals manage their condition effectively. These programs improve patient adherence to treatment plans and foster greater utilization of available therapies.
Economic growth and improved standards of living in Japan contribute to increased healthcare expenditure and access. As the economy grows and living standards improve, patients are more likely to afford out-of-pocket expenses for advanced psoriasis treatments. Higher disposable income supports the adoption of newer therapies and drives market growth. Economic prosperity leads to increased investment in healthcare infrastructure and services. This investment enhances access to high-quality care and advanced treatments, contributing to the expansion of the psoriasis drugs market.
Key Market Challenges
Regulatory and Market Access Barriers
Regulatory hurdles and market access issues present significant challenges to the growth of the psoriasis drugs market. The regulatory approval process for new psoriasis drugs in Japan can be lengthy and rigorous. Drug developers must navigate complex regulatory requirements and demonstrate robust clinical evidence to gain approval, which can delay market entry and affect the availability of innovative treatments.Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires extensive clinical evidence to support the safety and efficacy of new drugs. Meeting these high standards can be resource-intensive and time-consuming for pharmaceutical companies. Even after gaining regulatory approval, drugs may face delays in market access due to negotiation processes with healthcare authorities and insurance providers. These delays can impact the timely availability of new treatments and influence market dynamics.
Limited Patient Awareness and Education
Limited patient awareness and education about psoriasis and available treatments can restrict market growth by affecting treatment uptake. Many patients may not be fully aware of psoriasis treatments or the benefits of advanced therapies. This lack of awareness can lead to underutilization of available treatments and delayed diagnosis, impacting market demand.There may be gaps in patient education regarding the management of psoriasis and the importance of adhering to prescribed therapies. Addressing these educational gaps is crucial for improving patient outcomes and fostering market growth. Psoriasis is often associated with social stigma and psychological distress. Patients may be reluctant to seek treatment due to embarrassment or fear of discrimination, which can limit market growth and affect treatment adherence.
Key Market Trends
Advancements in Biologic Therapies
Biologic therapies represent a significant trend in the treatment of psoriasis, driven by continuous innovation and development of new biologic drugs. The development of next-generation biologics, such as IL-17 inhibitors and IL-23 inhibitors, offers more targeted and effective treatment options. These biologics provide improved efficacy and safety profiles, addressing the unmet needs of patients who do not respond well to traditional therapies.Advances in genomics and molecular biology are paving the way for personalized medicine approaches in psoriasis treatment. By understanding individual genetic profiles and disease mechanisms, healthcare providers can tailor biologic therapies to achieve optimal outcomes for each patient. The introduction of long-acting biologic formulations reduces the frequency of administration, enhancing patient convenience and adherence to treatment. These formulations are particularly beneficial for chronic conditions like psoriasis, where long-term management is crucial.
Integration of Digital Health Technologies
The integration of digital health technologies is revolutionizing the management of psoriasis, driving market growth through improved patient care and engagement. Telemedicine platforms enable remote consultations and monitoring of psoriasis patients, enhancing access to dermatological care. This is particularly important in Japan, where geographic barriers can limit access to specialized healthcare providers.Mobile health applications offer tools for tracking symptoms, medication adherence, and treatment outcomes. These apps empower patients to manage their condition more effectively and provide valuable data to healthcare providers for personalized treatment adjustments. AI-driven tools assist in diagnosing and monitoring psoriasis by analyzing skin images and clinical data. AI algorithms can predict disease flares and treatment responses, enabling proactive and timely interventions.
Increased Focus on Patient-Centric Care
There is a growing emphasis on patient-centric care in the psoriasis treatment paradigm, which is reshaping the market by prioritizing patient needs and experiences. Comprehensive education and support programs help patients understand their condition and treatment options. These programs enhance patient engagement, improve adherence to therapies, and lead to better health outcomes.Recognizing the impact of psoriasis on overall well-being, there is an increasing focus on holistic treatment approaches that address both physical and psychological aspects of the disease. Integrating mental health support and lifestyle interventions with medical treatment provides a more comprehensive care model. Patient advocacy groups are playing a crucial role in shaping healthcare policies and treatment guidelines. Their involvement ensures that patient perspectives are considered in decision-making processes, leading to more patient-centric care and better alignment of treatment strategies with patient needs.
Segmental Insights
Therapeutic Class Insights
Based on the category of Therapeutic Class, the tumor necrosis factor (TNF)-inhibitors segment emerged as the dominant in the market for Japan Psoriasis Drugs in 2024. TNF-inhibitors, such as infliximab, etanercept, and adalimumab, are highly effective in managing moderate to severe psoriasis. These biologic drugs target and neutralize TNF-alpha, a key cytokine involved in the inflammatory process of psoriasis. Their superior efficacy in reducing psoriasis symptoms and achieving rapid clinical improvement has made them a preferred choice among healthcare providers and patients.Leading dermatological associations and clinical guidelines in Japan endorse TNF-inhibitors as a first-line treatment for moderate to severe psoriasis. These endorsements are based on extensive clinical trials and real-world evidence demonstrating the long-term safety and efficacy of TNF-inhibitors. The strong backing from the medical community significantly boosts their adoption and usage. TNF-inhibitors are suitable for a wide range of psoriasis patients, including those who have not responded adequately to conventional systemic therapies or topical treatments. This broad eligibility criteria expands the patient base for TNF-inhibitors, contributing to their dominant market share. Additionally, these drugs can be used in patients with comorbid conditions, making them versatile treatment options.
The TNF-inhibitors segment benefits from continuous innovation and the introduction of new formulations and delivery methods. For instance, advancements in subcutaneous injection devices and extended-release formulations enhance patient convenience and adherence to therapy. These innovations improve patient outcomes and satisfaction, reinforcing the dominance of TNF-inhibitors in the market. TNF-inhibitors have a well-established safety profile, with manageable side effects. Long-term studies and post-marketing surveillance have confirmed their safety, which boosts confidence among healthcare providers and patients. The favorable risk-benefit ratio of TNF-inhibitors makes them a reliable choice for long-term management of psoriasis.
Pharmaceutical companies in Japan offer comprehensive patient support programs for TNF-inhibitor therapies. These programs include financial assistance, educational resources, and adherence support, which help patients manage their treatment effectively. Such initiatives reduce the financial burden on patients and improve treatment adherence, thereby driving market growth for TNF-inhibitors. These factors collectively contribute to the growth of this segment.
Regional Insights
Kanto emerged as the dominated in the Japan Psoriasis Drugs market in 2024, holding the largest market share in terms of value. The Kanto region, which includes Tokyo, Yokohama, and Kawasaki, is the most populous and urbanized area in Japan. This high population density translates to a larger patient pool for psoriasis treatment. Urbanization also leads to higher healthcare awareness and better access to medical facilities, driving demand for psoriasis drugs. Kanto boasts some of the most advanced healthcare facilities in Japan, including top-tier hospitals and research institutions. These facilities are equipped with the latest medical technologies and treatment protocols, enabling them to offer cutting-edge psoriasis treatments.The presence of renowned healthcare institutions attracts patients from across the country seeking high-quality care, further boosting the demand for psoriasis drugs in the region. As the economic center of Japan, Kanto has a higher average income level compared to other regions. This economic prosperity allows residents to afford advanced and often expensive psoriasis treatments. Additionally, the concentration of pharmaceutical companies and research institutions in Kanto fosters innovation and the development of new and effective psoriasis drugs, which further strengthens the market.
Kanto is home to numerous pharmaceutical companies, both domestic and multinational. These companies engage in extensive research and development activities, leading to the introduction of innovative psoriasis treatments. The region's strong pharmaceutical industry not only supplies the local market but also drives exports, reinforcing its dominance in the psoriasis drugs market. The Kanto region hosts a vibrant medical community with numerous dermatologists specializing in psoriasis treatment. Frequent medical conferences, seminars, and workshops held in the region promote knowledge sharing and the adoption of best practices in psoriasis management.
This strong medical community ensures that patients in Kanto receive the most up-to-date and effective treatments, thereby increasing the demand for advanced psoriasis drugs. The Japanese government, recognizing the importance of addressing chronic conditions like psoriasis, provides substantial support for healthcare initiatives in Kanto. Policies that encourage pharmaceutical innovation, coupled with healthcare reforms aimed at improving patient access to medications, contribute to the region’s leadership in the psoriasis drugs market. Government-funded programs and subsidies make advanced treatments more accessible to patients, driving market growth.
Key Market Players
- Eli Lilly & Company
- Pfizer Inc.
- Janssen Pharmaceuticals, Inc.
- Bristol-Myers Squibb Company
- Takeda Pharmaceutical Company Limited
- Novartis AG
- Amgen Inc
- Abbvie Inc
- AstraZeneca
- Boeringer Ingelheim International GmbH
Report Scope:
In this report, the Japan Psoriasis Drugs Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Japan Psoriasis Drugs Market, By Therapeutics Class:
- Tumor Necrosis Factor Inhibitor
- Interleukin Inhibitors
- Others
Japan Psoriasis Drugs Market, By Treatment:
- Topicals
- Systemic
- Biologics
Japan Psoriasis Drugs Market, By Region:
- Hokkaido
- Tohoku
- Kanto
- Chubu
- Kansai
- Chugoku
- Shikoku
- Kyushu
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Japan Psoriasis Drugs Market.Available Customizations:
Japan Psoriasis Drugs market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Eli Lilly & Company
- Pfizer Inc.
- Janssen Pharmaceuticals, Inc.
- Bristol-Myers Squibb Company
- Takeda Pharmaceutical Company Limited
- Novartis AG
- Amgen Inc
- Abbvie Inc
- AstraZeneca
- Boeringer Ingelheim International GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 82 |
| Published | September 2024 |
| Forecast Period | 2024 - 2030 |
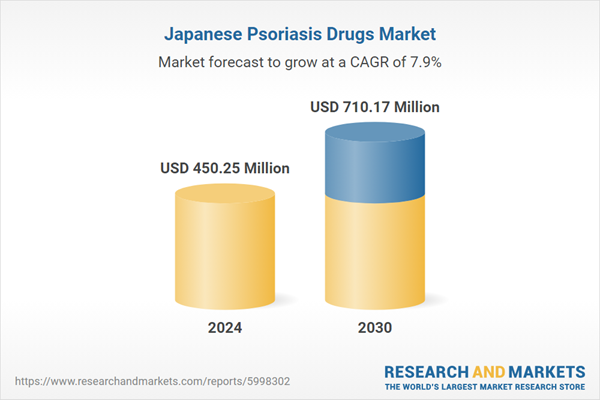
| Estimated Market Value ( USD | $ 450.25 Million |
| Forecasted Market Value ( USD | $ 710.17 Million |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 10 |