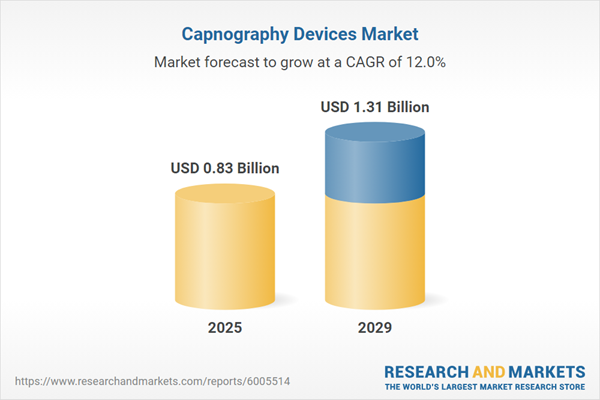
The capnography devices market size has grown rapidly in recent years. It will grow from $0.74 billion in 2024 to $0.83 billion in 2025 at a compound annual growth rate (CAGR) of 12.3%. The growth in the historic period can be attributed to the increasing incidence of respiratory diseases, advancements in technology, enhanced patient monitoring, regulatory support and guidelines, and the aging population.
The capnography devices market size is expected to see rapid growth in the next few years. It will grow to $1.31 billion in 2029 at a compound annual growth rate (CAGR) of 12%. The growth in the forecast period can be attributed to increasing surgical procedures, growing awareness and training programs, regulatory support and guidelines, and expanding applications. Major trends in the forecast period include increasing healthcare expenditure, growing focus on patient safety, rising awareness and training programs, expansion in home healthcare, and increasing use in intensive care units (ICUs).
The increasing prevalence of respiratory diseases is expected to drive the growth of the capnography devices market in the coming years. Respiratory diseases encompass a range of conditions that impact the lungs and respiratory system, such as asthma, chronic obstructive pulmonary disease (COPD), pneumonia, and lung cancer, which hinder breathing and can lead to severe health issues. The rising incidence of respiratory diseases is attributed to factors such as worsening air pollution, higher smoking rates, and aging populations. Capnography devices play a crucial role in monitoring and assessing patients' ventilation status, enabling the detection of abnormalities in real time. For example, in June 2024, the Australian Institute of Health and Welfare, an Australian health agency, reported that approximately 8.5 million Australians, or 34% of the population, were living with chronic respiratory conditions. This includes an estimated 2.8 million individuals (11% of the population) with asthma and 638,000 people (2.5% of the population) suffering from COPD. As a result, the growing prevalence of respiratory diseases is fueling the demand for capnography devices.
Major companies in the capnography devices market are intensifying their efforts to develop advanced pilot studies to secure a competitive advantage. Pilot studies are preliminary research efforts designed to assess the feasibility, effectiveness, and potential impact of these devices in clinical environments. For example, in March 2024, TidalSense, a UK-based company specializing in respiratory devices, launched a pilot study to evaluate its innovative N-Tidal device for diagnosing asthma in young children. This study, conducted in collaboration with the University of Nottingham and Nottingham University Hospitals NHS Trust, will include 75 children under five who are experiencing asthma and viral wheezing. The N-Tidal device employs capnography technology to measure carbon dioxide levels during normal breathing, thus bypassing the need for complex spirometry tests that are often challenging for young children, potentially leading to inaccurate diagnoses and extended hospital stays.
In June 2022, Medtronic plc, an Ireland-based medical device company, collaborated with GE HealthCare Technologies Inc. to integrate Medtronic's Microstream capnography and INVOS regional oximetry technologies into GE Healthcare's CARESCAPE precision monitoring platform. This collaboration enables comprehensive patient monitoring before, during, and after procedures, enhancing patient safety and outcomes. GE HealthCare Technologies Inc. is a US-based medical technology company.
Major companies operating in the capnography devices market are Medtronic PLC, Becton Dickinson and Company (BD), GE HealthCare Technologies Inc., Medline Industries Inc., Philips Healthcare, Mindray Medical International Limited, Dräger, Masimo Corporation, Nihon Kohden Corporation, Spacelabs Healthcare, Vyaire Medical, ZOLL Medical Corporation, Edan Instruments Inc., Weinmann Emergency Medical Technology GmbH + Co. KG, Salter Labs Inc., MGC Diagnostics Corporation, Criticare Systems Inc., Opto Circuits Limited, Diamedica Ltd., Infinium Medical Inc.
North America was the largest region in the capnography devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the capnography devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the capnography devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Capnography devices are medical instruments designed to measure and monitor the levels of carbon dioxide (CO2) in a patient's exhaled breath. They offer real-time insights into respiratory status, aid in identifying respiratory conditions, ensure adequate ventilation, and assist in managing anesthesia during surgeries.
The primary component types of capnography devices are original equipment manufacturer (OEM) modules and other components. OEM modules are produced by one company and incorporated into final products made by another company. These devices come in hand-held, stand-alone, and multi-parameter forms and utilize technologies such as mainstream, sidestream, and microstream. Capnography devices are applied in areas including emergency medicine, pain management, procedural sedation, and critical care, among others. They are used by a range of end-users, including hospitals, ambulatory care centers, and other facilities.
The capnography devices market research report is one of a series of new reports that provides capnography devices market statistics, including capnography devices industry global market size, regional shares, competitors with a capnography devices market share, detailed capnography devices market segments, market trends and opportunities, and any further data you may need to thrive in the capnography devices industry. This capnography devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The capnography devices market consists of sales of carbon dioxide (CO2) sensors, sampling lines, and display units. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Capnography Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on capnography devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for capnography devices ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The capnography devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Component: Original Equipment Manufacturer (OEM) Modules; Other Components2) By Product: Hand-held; Stand-alone; Multi-parameter
3) By Technology: Mainstream; Sidestream; Microstream
4) By Application: Emergency Medicine; Pain Management; Procedural Sedation; Critical Care; Other Applications
5) By End-use: Hospitals; Ambulatory Care; Other End-Uses
Subsegments:
1) By Original Equipment Manufacturer (OEM) Modules: Sensors; Actuators; Microcontrollers; Power Modules2) By Other Components: Connectors; Cables; Switches; Enclosures
Key Companies Mentioned: Medtronic PLC; Becton Dickinson and Company (BD); GE HealthCare Technologies Inc.; Medline Industries Inc.; Philips Healthcare
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Capnography Devices market report include:- Medtronic PLC
- Becton Dickinson and Company (BD)
- GE HealthCare Technologies Inc.
- Medline Industries Inc.
- Philips Healthcare
- Mindray Medical International Limited
- Dräger
- Masimo Corporation
- Nihon Kohden Corporation
- Spacelabs Healthcare
- Vyaire Medical
- ZOLL Medical Corporation
- Edan Instruments Inc.
- Weinmann Emergency Medical Technology GmbH + Co. KG
- Salter Labs Inc.
- MGC Diagnostics Corporation
- Criticare Systems Inc.
- Opto Circuits Limited
- Diamedica Ltd.
- Infinium Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 0.83 Billion |
| Forecasted Market Value ( USD | $ 1.31 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |