Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The expansion of healthcare facilities and the introduction of innovative devices are enhancing treatment accessibility. Supportive government initiatives and insurance coverage for varicose vein treatments further stimulate market demand. The growing trend towards aesthetic procedures and cosmetic surgery is encouraging individuals to seek interventions for both health and cosmetic reasons, thus broadening the market scope. Overall, a combination of demographic shifts, technological innovations, and increased consumer awareness is fueling the expansion of the United States Varicose Vein Treatment Devices Market.
Key Market Drivers
Rising Prevalence of Venous Disorders
The increasing incidence of venous disorders, particularly varicose veins, is a significant driver for the United States Varicose Vein Treatment Devices Market. This trend is largely influenced by several interrelated factors, including an aging population, sedentary lifestyles, and rising obesity rates. As the population ages, the risk of developing varicose veins escalates due to the natural decline in vascular health and elasticity. Older adults often experience weakened vein walls and impaired circulation, making them more susceptible to venous disorders. Sedentary lifestyles further exacerbate the prevalence of varicose veins.With the modern workforce increasingly engaged in desk jobs and reduced physical activity, the lack of movement can lead to poor blood circulation in the legs. Prolonged sitting or standing increases venous pressure, contributing to the formation of varicose veins. The trend of sedentary behavior is often accompanied by weight gain, leading to obesity, which is another critical risk factor for developing venous disorders. Excess weight places additional strain on the veins, worsening symptoms and prompting individuals to seek treatment. The American Vein & Lymphatic Society Patient Reported Outcome Venous Registry began collecting data in 2014 and is one of only two national registries dedicated to chronic venous disorders.
A review of over 270,000 unique patient records was conducted, of which 163,027 (60%) had accompanying duplex ultrasound scans, representing 1,794 unique patients (1,879 limbs) with a leg wound incidence of 1.1%. Among these patients, 55.4% were men and 44.6% were women. Group S consisted of patients with isolated superficial pathology (n = 1,291; 68.7%), Group M included those with mixed superficial and deep pathology (n = 238; 12.7%), Group D comprised patients with isolated deep vein pathology (n = 58; 3.1%), and Group N included those with leg wounds but no venous pathology (n = 292; 15.5%). The rVCSS scores for Groups S and M were significantly higher than those for Group N.
In Group S, the most common patterns involved the great saphenous vein (GSV) above the knee (54.8%), the small saphenous vein (30.7%), and the anterior accessory GSV (14.4%), with an average of 1.45 vessels eligible for ablation per limb. In Group M, the dominant patterns included the GSV above the knee (61.7%), the small saphenous vein (26.2%), and the anterior accessory GSV (12.1%), with an average of 1.52 axial segments per limb. Among the 84.4% of patients with venous ulcers, duplex ultrasound analysis indicated that 97% had surgically correctable disease.
As more people become aware of the symptoms and complications associated with varicose veins, the demand for effective treatment options rises. Patients are more likely to consult healthcare providers for evaluation and intervention as they become informed about the potential health risks tied to untreated varicose veins, such as chronic pain, skin changes, and more severe complications like venous ulcers or thrombophlebitis.
This growing patient interest drives healthcare providers to expand their offerings, including a broader range of varicose vein treatment devices. In response to this demand, manufacturers are motivated to innovate and enhance their product lines. The competitive landscape encourages the development of new technologies and techniques, focusing on minimally invasive procedures that offer shorter recovery times and improved outcomes. For example, advancements in laser and radiofrequency ablation techniques have transformed traditional treatment approaches, making them more appealing to patients who prioritize comfort and rapid recovery.
Increased Awareness and Education
Public awareness regarding the symptoms and risks associated with varicose veins has significantly increased in recent years, driven by comprehensive educational campaigns and heightened media coverage. These initiatives have played a crucial role in informing the general public about what varicose veins are, their potential complications, and the various treatment options available. As a result, individuals are now more vigilant about their vascular health, leading to an increase in consultations with healthcare providers for evaluation and treatment. One of the most impactful aspects of these awareness campaigns is their ability to demystify the condition.Many people previously viewed varicose veins as a mere cosmetic issue, not realizing the underlying health risks they can pose, such as chronic pain, skin changes, and even life-threatening conditions like deep vein thrombosis. Educational efforts have effectively communicated that early intervention is crucial in preventing these complications, encouraging patients to seek medical attention sooner rather than later. In September 2024, USA Vein Clinics announced the opening of its state-of-the-art clinic at Union City, NJ. This new facility is equipped with advanced technology, enabling experienced vein specialists to effectively address symptoms of chronic vein insufficiency (CVI), which is the underlying cause of varicose veins and spider veins.
As patients become more informed, their willingness to pursue treatment options grows. They are now more likely to recognize the symptoms of varicose veins - such as swelling, heaviness, and aching in the legs - and understand that these symptoms warrant medical evaluation. This increased patient engagement not only benefits individuals by promoting timely treatment but also drives demand for a wider range of treatment modalities. In response to this growing demand, healthcare providers are increasingly investing in advanced treatment devices to enhance their service offerings. This investment is fueled by the understanding that patients expect effective, minimally invasive options with shorter recovery times. As healthcare facilities upgrade their equipment and adopt cutting-edge technologies, they position themselves to better meet the needs of an informed patient population.
Growing Interest in Aesthetic Procedures
The cosmetic aspect of varicose vein treatment is gaining significant traction, reflecting a broader societal shift where individuals increasingly seek procedures that enhance not only their health but also their appearance. This dual focus on health and aesthetics has transformed varicose vein treatments from purely medical interventions into sought-after cosmetic procedures. Many patients are motivated by the desire for clear, healthy-looking legs, which has become an essential aspect of their overall self-image and confidence.As awareness of the negative impact that varicose veins can have on one’s appearance grows, more individuals are pursuing treatments to eliminate these unsightly veins. Varicose veins can be a source of self-consciousness, particularly for those who prioritize their physical appearance in social and professional settings. The growing emphasis on body positivity and personal aesthetics in popular culture has further propelled this trend, encouraging patients to seek out solutions that align with evolving beauty standards.
This shift in consumer behavior has led to an expansion of the market for varicose vein treatment devices. Manufacturers are responding to the rising demand by developing innovative solutions that address both the functional and aesthetic aspects of treatment. For instance, advancements in minimally invasive techniques, such as endovenous laser therapy (EVLT) and sclerotherapy, are not only effective in treating the underlying medical issues but also deliver satisfactory cosmetic results. These procedures allow patients to achieve a more youthful appearance without significant downtime, making them increasingly appealing.
Increased Focus on Preventive Healthcare
A notable shift toward preventive healthcare is significantly influencing the United States Varicose Vein Treatment Devices Market. This change in focus highlights the increasing recognition among healthcare providers of the importance of early diagnosis and timely intervention for venous disorders. Traditionally, many patients sought treatment only after experiencing severe symptoms or complications. However, healthcare professionals are now actively promoting the idea that early detection can lead to better outcomes and more effective management of varicose veins.This emphasis on prevention encourages patients to engage in regular check-ups and screenings, fostering a proactive approach to their health. Healthcare providers are implementing awareness campaigns that inform patients about the risk factors associated with varicose veins, such as family history, age, obesity, and lifestyle choices. By educating patients about these risks, providers are empowering them to take charge of their health and seek evaluations before symptoms escalate.
As a result of this proactive mindset, more individuals are being diagnosed with varicose veins at earlier stages, which allows for timely and less invasive treatment options. Early intervention not only mitigates the progression of the condition but also enhances the effectiveness of available treatments, as less advanced cases often respond better to conservative therapies. This proactive approach to healthcare creates a significant increase in demand for effective treatment devices, as more patients are opting for intervention before their conditions worsen.
Key Market Challenges
High Competition and Market Saturation
The United States Varicose Vein Treatment Devices Market is characterized by intense competition and increasing market saturation. Numerous established companies and emerging startups offer a wide range of treatment devices and technologies, leading to a crowded marketplace. This competition drives prices down, squeezing profit margins for manufacturers and requiring them to continuously innovate to differentiate their products.In such a saturated market, establishing a strong brand presence becomes crucial. Manufacturers must invest heavily in marketing, sales, and customer education to capture market share. This often necessitates significant financial resources, which can be challenging for smaller companies or startups. As competition intensifies, the pressure to innovate increases, leading companies to prioritize research and development efforts. However, this need for constant innovation can strain resources and divert attention from other important business functions.
The competition also extends to healthcare providers, who are increasingly adopting a consumer-centric approach. Patients today have access to vast amounts of information, making them more discerning when selecting healthcare providers and treatment options. Providers must differentiate their offerings not just through the technologies they use but also through patient experience, outcomes, and reputation. This dynamic can force manufacturers to align their product development with the evolving needs and preferences of both patients and healthcare providers.
Cost Barriers and Insurance Coverage Issues
Cost remains a significant barrier in the United States Varicose Vein Treatment Devices Market. Despite advancements in technology and treatment options, many patients face high out-of-pocket expenses for procedures, especially if their insurance plans do not provide adequate coverage for varicose vein treatments. This financial burden can deter patients from seeking timely interventions, leading to a reluctance to invest in necessary medical care. Insurance coverage for varicose vein treatments can vary widely, creating confusion for patients navigating their options. Some procedures may be deemed medically necessary, while others are classified as elective or cosmetic, impacting their coverage. Patients may be reluctant to pursue treatments that are not covered by their insurance, resulting in delayed diagnosis and treatment, which can worsen their conditions over time.The economic environment plays a role in influencing patients’ willingness to pay for elective procedures. Economic downturns can lead to increased financial uncertainty, prompting patients to prioritize essential healthcare services over elective or cosmetic treatments. This trend can significantly impact the overall demand for varicose vein treatment devices. Manufacturers must consider these cost barriers when developing and marketing their products. Creating value propositions that highlight the long-term benefits and cost savings associated with early intervention can help encourage patients and providers to invest in advanced treatment devices. Collaboration with insurance providers to enhance coverage for varicose vein treatments may improve patient access and boost demand.
Key Market Trends
Technological Advancements
Technological innovation plays a pivotal role in the expansion of the United States Varicose Vein Treatment Devices Market, fundamentally transforming how healthcare providers approach the management of venous disorders. Recent advancements in minimally invasive techniques, such as endovenous laser therapy (EVLT) and radiofrequency ablation (RFA), have revolutionized the treatment landscape. These techniques have emerged as preferred options due to their effectiveness, reduced invasiveness, and improved patient experience.One of the standout features of EVLT and RFA is their ability to target varicose veins without the need for extensive surgical procedures. Traditional methods, such as vein stripping, often involved significant discomfort, longer recovery periods, and increased risk of complications. In contrast, minimally invasive techniques utilize localized anesthesia and can often be performed on an outpatient basis, allowing patients to return to their daily activities almost immediately. This convenience is a significant selling point, as it addresses the common patient concerns related to time off work and the overall impact on their lifestyles.
These advanced techniques reduce patient discomfort significantly. Studies have shown that patients undergoing EVLT or RFA report lower levels of pain compared to those receiving traditional surgical treatments. The use of smaller incisions and precise application of energy to close off problem veins minimizes tissue damage and speeds up healing. This patient-centered approach not only enhances satisfaction but also drives the overall demand for these innovative treatment options.
Expansion of Healthcare Facilities
The expansion of healthcare facilities across the United States plays a crucial role in driving the growth of the United States Varicose Vein Treatment Devices Market. As more outpatient clinics, ambulatory surgical centers, and specialized vein treatment centers emerge, patient access to care is significantly enhanced. This increased accessibility is vital for individuals suffering from venous disorders, as it provides them with a wider range of options for evaluation and treatment.One of the key benefits of this expansion is the ability to bring services closer to patients’ homes. Previously, many individuals had to travel long distances to receive specialized care for varicose veins, which could deter them from seeking timely treatment. The establishment of local clinics and centers reduces logistical barriers, encouraging more patients to pursue necessary evaluations and interventions. This geographical convenience is particularly important for older adults, who may face mobility challenges or require frequent visits for ongoing management.
The proliferation of healthcare facilities is also accompanied by the hiring of skilled professionals trained in the latest techniques and technologies for treating varicose veins. As the demand for these services grows, clinics are investing in recruiting specialized staff, including vascular surgeons, interventional radiologists, and trained nurses. These professionals bring expertise in minimally invasive procedures, such as endovenous laser therapy (EVLT) and radiofrequency ablation (RFA), which are increasingly preferred by patients for their effectiveness and reduced recovery times.
The presence of skilled practitioners enhances the overall quality of care, leading to better patient outcomes. As healthcare providers implement advanced techniques, patients are more likely to experience successful results and fewer complications, which can boost the reputation of these facilities. Positive patient experiences often translate into word-of-mouth referrals and increased patient retention, further driving demand for varicose vein treatments. The competitive landscape created by the growth of healthcare facilities encourages providers to invest in advanced treatment devices. To attract patients, clinics must differentiate themselves by offering cutting-edge technologies and comprehensive care options. This competition fosters an environment where innovation thrives, leading to the development and adoption of new devices and techniques that improve treatment efficacy and patient satisfaction.
Segmental Insights
Type Insights
Based on the Type, endovenous ablation techniques, particularly endovenous laser therapy (EVLT) and radiofrequency ablation (RFA), are currently dominating the United States varicose vein treatment devices market. This dominance can be attributed to several factors, including the effectiveness, minimally invasive nature, and favorable patient outcomes associated with these procedures. Endovenous ablation techniques have revolutionized the management of varicose veins by offering patients a less invasive alternative to traditional surgical options such as ligation and stripping.The latter, which involves the surgical removal of affected veins, often requires general anaesthesia and results in significant discomfort and longer recovery times. In contrast, endovenous ablation is typically performed on an outpatient basis using local anaesthesia, which greatly enhances patient comfort and reduces the time away from daily activities. Patients can often resume normal activities shortly after the procedure, which makes it an appealing choice for those who prioritize convenience and a swift return to their routine.
Another critical factor contributing to the dominance of endovenous ablation is its high success rate. Studies have shown that these techniques effectively close off varicose veins with minimal risk of complications. The targeted approach of endovenous ablation allows for precise treatment of problematic veins while preserving surrounding tissue, which is not always possible with more invasive surgical methods. This effectiveness leads to improved patient satisfaction and encourages healthcare providers to adopt these techniques more widely.
End Use Insights
Based on the End Use, United States varicose vein treatment devices market is primarily dominated by hospitals, with clinics and ambulatory care centers playing significant but secondary roles. This dominance can be attributed to several factors, including the comprehensive range of services offered, advanced technological capabilities, and the availability of specialized healthcare professionals.Hospitals are equipped with state-of-the-art facilities and advanced medical technology, allowing them to provide a wide variety of treatment options for varicose veins, such as endovenous laser therapy (EVLT), sclerotherapy, and radiofrequency ablation. These procedures often require sophisticated equipment and experienced medical personnel, which hospitals are uniquely positioned to offer. The presence of multidisciplinary teams, including vascular surgeons, radiologists, and anesthesiologists, enhances patient safety and care quality, making hospitals a preferred choice for more complex cases.
Hospitals have the ability to manage a higher volume of patients, which not only increases their market share but also enables them to invest in the latest treatment devices. This investment is crucial, as the rapid advancement of technology in the healthcare sector continually introduces new devices and techniques. Hospitals are more likely to stay at the forefront of these developments, allowing them to offer innovative treatment options that can attract more patients. Many patients prefer the reassurance of receiving treatment in a hospital setting, particularly when dealing with a condition like varicose veins, which can have complications if not treated properly.
Regional Insights
In the United States varicose vein treatment devices market, the South region is currently dominating, driven by a combination of demographic factors, healthcare infrastructure, and increasing awareness of venous disorders. This dominance can be attributed to the region's larger population base, higher prevalence of obesity, and the rising number of aging individuals, all of which contribute to a greater demand for varicose vein treatments.The South has experienced significant population growth in recent years, with states like Texas, Florida, and North Carolina seeing substantial increases in residents. This growth not only leads to a larger pool of potential patients but also enhances the market for healthcare services, including varicose vein treatments. The demographic composition of the South also plays a crucial role; the region has a higher proportion of individuals aged 50 and above, who are more susceptible to venous diseases. As awareness of the health risks associated with untreated varicose veins increases, more patients are seeking treatment options, driving demand for specialized devices and services.
The healthcare infrastructure in the South has been expanding rapidly. A growing number of hospitals and specialized clinics are investing in advanced varicose vein treatment technologies, such as endovenous laser therapy (EVLT) and radiofrequency ablation. This investment is essential, as it allows facilities to offer a wider range of treatment options that cater to different patient needs. Hospitals in the South are increasingly partnering with vascular specialists and employing advanced medical technologies, which enhances their ability to provide high-quality care. The presence of experienced healthcare professionals further strengthens the region's capacity to address the complexities of varicose vein treatments.
Key Market Players
- AngioDynamics, Inc.
- Medtronic PLC
- Teleflex Incorporated
- Sciton, Inc.
- Dornier MedTech America, Inc.
- Merit Medical Systems
- Boston Scientific Corporation
- Candela Corporation
- Becton, Dickinson and Company
Report Scope:
In this report, the United States Varicose Vein Treatment Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:United States Varicose Vein Treatment Devices Market, By Type:
- Endo venous Ablation
- Sclerotherapy
- Surgical Ligation & Stripping
United States Varicose Vein Treatment Devices Market, By End Use:
- Clinics
- Hospitals
- Ambulatory Care Centers
United States Varicose Vein Treatment Devices Market, By Region:
- North-East
- Mid-West
- West
- South
Competitive Landscape
Company Profiles: Detailed analysis of the major companies presents in the United States Varicose Vein Treatment Devices Market.Available Customizations:
United States Varicose Vein Treatment Devices Market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- AngioDynamics, Inc.
- Medtronic PLC
- Teleflex Incorporated
- Sciton, Inc.
- Dornier MedTech America, Inc.
- Merit Medical Systems
- Boston Scientific Corporation
- Candela Corporation
- Becton, Dickinson and Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 82 |
| Published | October 2024 |
| Forecast Period | 2023 - 2029 |
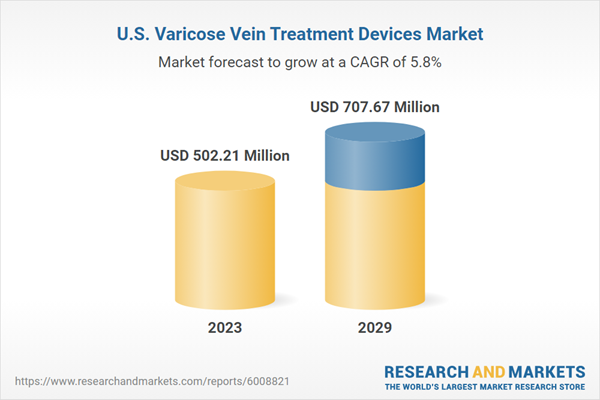
| Estimated Market Value ( USD | $ 502.21 Million |
| Forecasted Market Value ( USD | $ 707.67 Million |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | United States |
| No. of Companies Mentioned | 9 |