Global Hydroxychloroquine Market - Key Trends and Drivers Summarized
What is Hydroxychloroquine, and Why Has It Garnered Global Attention?
Hydroxychloroquine is a medication that was originally developed to treat malaria but has since become widely used in the management of autoimmune disorders such as lupus and rheumatoid arthritis. It works by modulating the immune system and reducing inflammation, making it an effective therapeutic option for these chronic conditions. Chemically, hydroxychloroquine is a derivative of chloroquine, another antimalarial drug, but it is considered to have a better safety profile and fewer side effects. In recent years, hydroxychloroquine has gained unprecedented global attention due to its controversial and widely debated use as a potential treatment for COVID-19. Early in the pandemic, some preliminary studies and anecdotal evidence suggested that it might help in reducing the severity of the virus by inhibiting viral replication and modulating the immune response. This sparked a surge in demand and global interest in the drug, with some countries stockpiling it and doctors prescribing it off-label. However, subsequent large-scale clinical trials and scientific investigations yielded mixed results, leading to significant debate and confusion about its efficacy. Regulatory agencies like the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA) initially issued emergency use authorizations but later revoked them, citing a lack of conclusive evidence and potential safety concerns. This ongoing scrutiny has kept hydroxychloroquine in the spotlight, not just in the medical community but also in public discourse, making it one of the most talked-about drugs of recent times.Who is Using Hydroxychloroquine, and What Are Its Main Medical Applications?
Hydroxychloroquine continues to be widely used in its approved indications, primarily as a disease-modifying antirheumatic drug (DMARD) for autoimmune conditions like lupus and rheumatoid arthritis. Patients suffering from these disorders often experience chronic inflammation and an overactive immune response, and hydroxychloroquine helps by modulating these processes to prevent flare-ups and manage symptoms. For lupus patients, in particular, the drug has proven to be invaluable, helping to control skin rashes, joint pain, and fatigue, and preventing organ damage associated with severe disease manifestations. Its use in rheumatoid arthritis is also well-documented, where it helps reduce joint swelling, pain, and the long-term damage caused by autoimmune activity. Beyond these established applications, hydroxychloroquine's role as an antimalarial agent remains relevant in regions where malaria is endemic, although it is now largely used for prevention and in specific cases where resistance to newer antimalarials is not a concern. The COVID-19 pandemic brought a surge of off-label use among both healthcare professionals and the general public, fueled by early reports suggesting potential antiviral properties. This off-label usage led to shortages and raised concerns about access for patients who depend on the drug for chronic conditions. Despite the controversies, hydroxychloroquine's immunomodulatory and anti-inflammatory properties have kept it under consideration for research in various other diseases, including Sjögren's syndrome and sarcoidosis. Thus, while its place in COVID-19 treatment has been largely debunked, hydroxychloroquine remains a critical medication for millions suffering from autoimmune diseases worldwide.What Challenges and Controversies Surround Hydroxychloroquine?
Hydroxychloroquine has been at the center of a highly charged and complex controversy, driven by a combination of scientific uncertainty, political dynamics, and public misinformation. One of the biggest challenges is the conflicting data surrounding its safety and efficacy for uses beyond its approved indications. Early in the COVID-19 pandemic, small observational studies and in vitro experiments suggested potential benefits, leading to a wave of off-label use. However, as larger randomized controlled trials were conducted, many failed to show any significant clinical benefits, and some even reported adverse effects, particularly cardiovascular complications like arrhythmias and QT prolongation. This inconsistency in results led to confusion among both healthcare providers and the public, eroding trust in scientific research and regulatory bodies. Adding to the confusion was the politicization of the drug, with high-profile endorsements and misinformation campaigns creating a polarized debate that further muddied public perception. The initial hype led to widespread hoarding, stockpiling, and in some cases, self-medication, which resulted in toxicity and hospitalizations. This situation posed significant challenges for patients who rely on hydroxychloroquine for chronic conditions like lupus and rheumatoid arthritis, as shortages disrupted their treatment regimens. Additionally, regulatory decisions regarding hydroxychloroquine have been inconsistent, with agencies such as the FDA and the European Medicines Agency (EMA) issuing and then retracting emergency use authorizations. This regulatory back-and-forth has raised broader questions about the process of scientific validation and the role of politics in public health decision-making. Despite these controversies, hydroxychloroquine's safety profile for its approved uses remains well-established, though its reputation has been undeniably affected by the turbulence of the past few years.What Factors Are Driving the Continued Interest in Hydroxychloroquine?
The growth in interest and research into hydroxychloroquine is driven by several factors related to its established therapeutic benefits, ongoing scientific inquiries, and the quest for cost-effective treatment options. One of the main drivers is its long-standing use as a safe and effective treatment for autoimmune disorders, making it a mainstay in the management of diseases like lupus and rheumatoid arthritis. For patients with these chronic conditions, hydroxychloroquine has proven to be a reliable medication that reduces disease activity and prevents complications, with a relatively favorable side effect profile compared to other immunosuppressive drugs. This established efficacy has sustained interest in exploring its potential applications in other immune-mediated diseases, such as Sjögren's syndrome and sarcoidosis, where inflammation and immune dysregulation play a key role. Another key factor driving interest is its affordability and widespread availability. As a generic drug, hydroxychloroquine is inexpensive compared to newer immunomodulatory or biologic therapies, making it an attractive option, particularly in low - and middle-income countries where healthcare resources are limited. Moreover, the controversies surrounding its use in COVID-19 have paradoxically fueled more research into its pharmacological properties, with some studies now focusing on understanding the exact mechanisms of action that contribute to its immunomodulatory effects. Researchers are looking into its impact on cytokine levels, potential benefits in preventing thrombotic complications, and its influence on various signaling pathways involved in immune response. As a result, hydroxychloroquine remains a subject of scientific inquiry, not just for its known applications, but also for its broader role in the pharmacological landscape of immunomodulatory treatments.Report Scope
The report analyzes the Hydroxychloroquine market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Disease Type (Malaria Disease, Rheumatoid Arthritis Disease, Other Disease Types); End-Use (Hospitals & Clinics End-Use, Homecare End-Use, Other End-Uses).
- Geographic Regions/Countries:World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Regional Analysis
Gain insights into the U.S. market, valued at $291.3 Million in 2024, and China, forecasted to grow at an impressive 6.2% CAGR to reach $268.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hydroxychloroquine Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hydroxychloroquine Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hydroxychloroquine Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam PLC, Amneal Pharmaceuticals, Inc., Apotex, Inc., Daicel Chiral Technologies (India) Pvt. Ltd., Dr. Reddy's Laboratories Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 56 companies featured in this Hydroxychloroquine market report include:
- Abcam PLC
- Amneal Pharmaceuticals, Inc.
- Apotex, Inc.
- Daicel Chiral Technologies (India) Pvt. Ltd.
- Dr. Reddy's Laboratories Ltd.
- Hetero Healthcare Ltd.
- Ipca Laboratories Ltd.
- Lifevision Healthcare
- Lupin Ltd.
- MilliporeSigma
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam PLC
- Amneal Pharmaceuticals, Inc.
- Apotex, Inc.
- Daicel Chiral Technologies (India) Pvt. Ltd.
- Dr. Reddy's Laboratories Ltd.
- Hetero Healthcare Ltd.
- Ipca Laboratories Ltd.
- Lifevision Healthcare
- Lupin Ltd.
- MilliporeSigma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 293 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
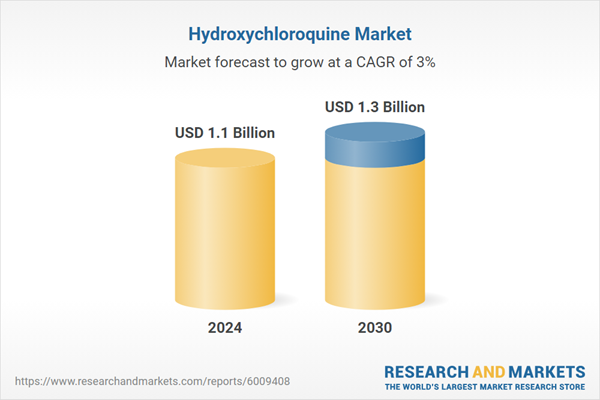
| Estimated Market Value ( USD | $ 1.1 Billion |
| Forecasted Market Value ( USD | $ 1.3 Billion |
| Compound Annual Growth Rate | 3.0% |
| Regions Covered | Global |