Global Gemcitabine Hydrochloride Market - Key Trends & Drivers Summarized
Why is Gemcitabine Hydrochloride Gaining Prominence in Cancer Treatment Protocols?
Gemcitabine Hydrochloride, a widely used chemotherapeutic agent, has become a cornerstone in the treatment of various cancers, including non-small cell lung cancer, pancreatic cancer, breast cancer, and ovarian cancer. Its efficacy as a nucleoside analog that interferes with DNA synthesis makes it a potent option for inhibiting tumor growth and proliferation. Over the past few years, there has been a marked increase in the adoption of Gemcitabine, driven by its effectiveness in combination therapy with other chemotherapeutic agents and targeted therapies. Studies have shown that combining Gemcitabine with platinum-based compounds, such as cisplatin, significantly improves patient outcomes, particularly in lung and bladder cancers. This has led to its inclusion in standard cancer treatment regimens across multiple oncology practices worldwide. Additionally, the relatively manageable side effect profile of Gemcitabine compared to other chemotherapies makes it a preferred option for elderly patients and those with comorbidities, further expanding its utility in clinical practice.Regionally, the demand for Gemcitabine Hydrochloride is predominantly high in developed regions like North America and Europe, where oncology research and healthcare infrastructure are more advanced. In these regions, the emphasis on personalized medicine and tailored treatment protocols has led to a steady uptake of Gemcitabine in combination therapies. However, emerging markets such as Asia-Pacific and Latin America are expected to experience the fastest growth, owing to the rising incidence of cancer, improved healthcare access, and increasing government initiatives to combat cancer. Pharmaceutical companies are leveraging this trend by expanding their presence in these regions and investing in local production facilities to reduce costs and improve distribution efficiency. As a result, the global market for Gemcitabine Hydrochloride is poised for robust growth, driven by its proven clinical benefits, expanding therapeutic applications, and growing acceptance in both mono and combination therapies.
What Innovations in Drug Delivery and Formulation Are Driving Gemcitabine Hydrochloride's Market Expansion?
Technological advancements in drug delivery and formulation are significantly shaping the Gemcitabine Hydrochloride market, making the drug more effective and easier to administer. Traditionally, Gemcitabine has been administered intravenously, which, although effective, comes with certain limitations such as inconvenience and potential for infusion-related complications. In response to these challenges, researchers and pharmaceutical companies are exploring alternative delivery mechanisms, including oral formulations, nanotechnology-based delivery systems, and localized drug delivery through transdermal patches or implants. These innovations are aimed at improving the bioavailability of Gemcitabine, reducing systemic toxicity, and enhancing patient compliance. For instance, nanotechnology-based delivery methods are being tested to ensure a more targeted approach, wherein the drug can be delivered directly to cancerous cells, minimizing exposure to healthy tissues and reducing side effects. Such advancements are expected to revolutionize the way Gemcitabine is administered, potentially making it suitable for a wider range of patients and indications.Another critical area of research and development is the formulation of Gemcitabine conjugates and prodrugs. These novel formulations are designed to overcome the rapid degradation of Gemcitabine in the bloodstream, thereby enhancing its therapeutic efficacy and reducing the frequency of administration. Prodrug approaches, which involve modifying the chemical structure of Gemcitabine to make it more stable, are being extensively studied for their potential to improve patient outcomes. Additionally, combination therapies using Gemcitabine with newer immunotherapeutic agents and targeted therapies are being explored to develop more comprehensive treatment regimens for various cancers. Such research is leading to new patents and regulatory approvals, enabling pharmaceutical companies to introduce next-generation formulations of Gemcitabine Hydrochloride in the market. The continuous innovation in drug delivery and formulation technologies is expected to significantly contribute to the expansion of the Gemcitabine Hydrochloride market in the coming years.
How Are Market Dynamics and Healthcare Policies Shaping the Adoption of Gemcitabine Hydrochloride?
The adoption of Gemcitabine Hydrochloride is being influenced by a complex interplay of market dynamics and healthcare policies, which vary significantly across different regions. In developed markets like the United States and Western Europe, the strong focus on evidence-based medicine and clinical guidelines ensures that Gemcitabine remains a key component in standard cancer treatment regimens. The presence of well-established healthcare infrastructure, coupled with favorable reimbursement policies, supports the widespread use of this chemotherapeutic agent. Additionally, cancer care pathways in these regions often prioritize newer and more effective treatment options, and Gemcitabine's proven track record in improving survival rates makes it a consistent choice for oncologists. Furthermore, the drug's inclusion in the National Comprehensive Cancer Network (NCCN) guidelines for multiple cancer types solidifies its status as a staple in oncology treatment protocols.In contrast, the adoption of Gemcitabine in emerging markets is influenced by factors such as affordability, availability, and healthcare infrastructure constraints. Many developing countries are experiencing an increasing burden of cancer due to lifestyle changes, population aging, and improved detection rates. However, the high cost of cancer treatments remains a significant barrier. To address these challenges, governments in countries like China, India, and Brazil are implementing policies aimed at reducing the cost of essential oncology drugs, including Gemcitabine, through subsidies, local manufacturing, and generic production. The growing presence of biosimilar versions of Gemcitabine is also helping to reduce costs and increase accessibility in these regions. Additionally, the expansion of healthcare coverage and increased investment in cancer care infrastructure are expected to support the broader adoption of Gemcitabine Hydrochloride in these markets. These dynamics indicate that, while developed markets continue to drive high-value demand, emerging regions represent substantial growth opportunities for the Gemcitabine Hydrochloride market.
What Are the Key Growth Drivers Fueling the Expansion of the Gemcitabine Hydrochloride Market?
The growth in the global Gemcitabine Hydrochloride market is driven by several factors, including the rising prevalence of cancer, advancements in drug formulations, and increasing acceptance of combination therapies. The escalating burden of cancer globally, particularly lung, pancreatic, and breast cancers, is one of the most significant drivers of the Gemcitabine market. The drug's ability to effectively target rapidly dividing cells and its efficacy in combination with other chemotherapeutic agents have made it a preferred choice for many oncologists. Furthermore, ongoing research into the use of Gemcitabine in combination with newer agents, such as checkpoint inhibitors and targeted therapies, is expanding its therapeutic scope and offering improved outcomes for patients. This has led to a surge in clinical trials and new approvals, which are further strengthening its market position. Additionally, the development of generic and biosimilar versions of Gemcitabine is making the drug more affordable, thereby increasing its adoption, especially in cost-sensitive markets.Another key growth driver is the rising healthcare expenditure in emerging economies, which is enabling greater access to cancer treatments. Countries like China and India, which are witnessing a surge in cancer incidence, are making significant investments in healthcare infrastructure and oncology care, creating new opportunities for the Gemcitabine Hydrochloride market. Pharmaceutical companies are responding to these trends by forming strategic alliances and investing in local production facilities to cater to the growing demand. Moreover, the increasing focus on early diagnosis and treatment of cancer, supported by government screening programs and awareness campaigns, is leading to higher detection rates and, consequently, increased usage of effective chemotherapeutic agents like Gemcitabine. Lastly, the inclusion of Gemcitabine in international cancer treatment guidelines and the strong support from leading health organizations are enhancing its credibility and driving its adoption across diverse healthcare settings, ultimately contributing to sustained market growth.
Report Scope
The report analyzes the Gemcitabine Hydrochloride market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Drug Type (Generic Drugs, Branded Drugs); Condition (Pancreatic Cancer, Breast Cancer, Ovarian Cancer, Non-Small Cell Lung Carcinoma (NSCLC), Other Conditions); End-Use (Hospitals End-Use, Cancer Centers End-Use, Other End-Uses).
- Geographic Regions/Countries:World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Generic Drugs segment, which is expected to reach US$694.3 Million by 2030 with a CAGR of a 5.3%. The Branded Drugs segment is also set to grow at 4.4% CAGR over the analysis period.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Gemcitabine Hydrochloride Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Gemcitabine Hydrochloride Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Gemcitabine Hydrochloride Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbexa, Accord Healthcare, Inc., BioCrick BioTech, Cayman Chemical Company, Dr. Reddy's Laboratories Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Gemcitabine Hydrochloride market report include:
- Abbexa
- Accord Healthcare, Inc.
- BioCrick BioTech
- Cayman Chemical Company
- Dr. Reddy's Laboratories Ltd.
- Eli Lilly and Company
- Fresenius Kabi USA
- LGC Standards
- LKT Laboratories, Inc.
- Merck KGaA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbexa
- Accord Healthcare, Inc.
- BioCrick BioTech
- Cayman Chemical Company
- Dr. Reddy's Laboratories Ltd.
- Eli Lilly and Company
- Fresenius Kabi USA
- LGC Standards
- LKT Laboratories, Inc.
- Merck KGaA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 380 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
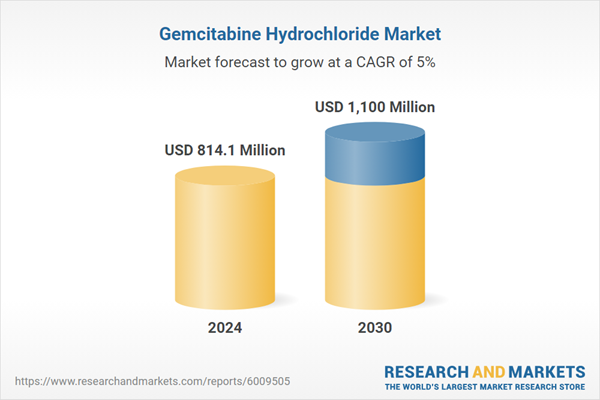
| Estimated Market Value ( USD | $ 814.1 Million |
| Forecasted Market Value ( USD | $ 1100 Million |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |