Pharmaceutical Stability And Storage Services Market Growth & Trends
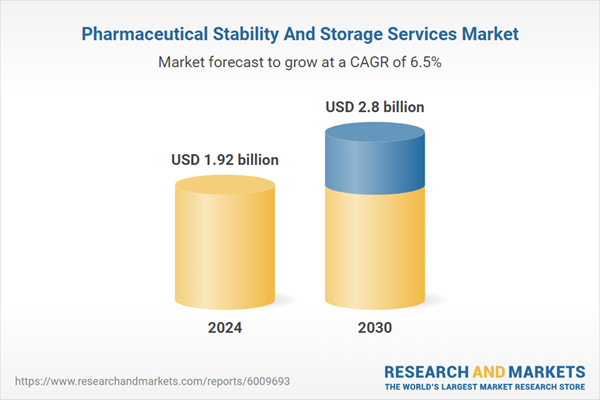
The global pharmaceutical stability and storage services market size is expected to reach USD 2.80 billion by 2030, registering a CAGR of 6.48% from 2025 to 2030. Stability and storage is a mandatory regulation in various regions. For instance, different regulatory authorities have different data requirements and testing rules for testing stability. Even though FDA and EMA follow the ICH guidelines for stability testing, they still have different microbiological limits for stability tests. This has improved the demand for stability testing outsourcing services and is likely to have a positive impact on the market.The COVID-19 pandemic had increased the demand for COVID-19 vaccines globally. The growing vaccine drive by the government authorities is likely to drive the demand for stability and storage of commercial COVID-19 vaccines. In recent years pharmaceutical R&D spending has improved significantly. The growing R&D spending is expected to improve the number of drugs in the clinical stage. Stability testing is required for the approval of each phase of a clinical trial. This is further driving the market growth. Moreover, Biosimilar drugs are highly similar copies of biologics and are very cheaper, as compared to biologics.
Biosimilar drugs are widely used in cancer, autoimmune diseases, and other diseases. This is contributing to the demand for biosimilar drugs and thereby is expected to drive the market demand. There has been a rise in several diseases post-COVID-19. For instance, according to a report published by Children’s National Hospital- pediatric research and clinical innovations center, a study was performed on 737 youths who were diagnosed with diabetes, and it found an increased incidence of pediatric Type 1 Diabetes (T1D) by 15.2% and Type 2 Diabetes (T2D) increased by 182% between March 11, 2018, and March 10, 2021. The rise in the disease incidence is expected to improve, drug production, which is likely to drive the market demand.
Pharmaceutical Stability And Storage Services Market Report Highlights
- Stability testing services segment dominated the pharmaceutical stability and storage industry with a share of 73.5% in 2024. The growth of the segment is mainly due to its important role in regulatory compliance, product safety, and efficacy.
- Small molecule segment dominated the services market in 2024. There have been significant advancements in small molecule drug development.
- North America pharmaceutical stability and storage services market dominated the market and accounted for a 40.0% share in 2024. The growth in the region is attributed to the presence of several pharmaceutical and biotech companies in the region, coupled with increasing research and development activities are driving the regions market growth.
- Asia Pacific pharmaceutical stability & storage services market is expected to grow at the highest CAGR over the forecast period. Asia Pacific is becoming a major center for clinical trials due to its large and diverse patient population and favorable regulatory environments.
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 2 business days.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Market Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. Internal Database
1.4.3. Secondary Sources
1.4.4. Primary Research
1.5. Market Formulation & Validation
1.6. Model Details
1.6.1. Commodity Flow Analysis
1.6.2. Bottom-up Approach
1.7. List of Secondary Sources
1.8. List of Abbreviations
1.9. Objectives
1.9.1. Objective 1
1.9.2. Objective 2
1.2. Market Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. Internal Database
1.4.3. Secondary Sources
1.4.4. Primary Research
1.5. Market Formulation & Validation
1.6. Model Details
1.6.1. Commodity Flow Analysis
1.6.2. Bottom-up Approach
1.7. List of Secondary Sources
1.8. List of Abbreviations
1.9. Objectives
1.9.1. Objective 1
1.9.2. Objective 2
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Snapshot
2.3. Competitive Insights Landscape
2.2. Segment Snapshot
2.3. Competitive Insights Landscape
Chapter 3. Pharmaceutical Stability & Storage Services Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook.
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. High R&D Spending of Pharmaceutical & Biotechnology Companies
3.2.1.2. Growing Demand for Outsourcing Stability Testing Due to High Complexity Associated with Drug Stability
3.2.1.3. Growing Demand for Biosimilars
3.2.1.4. Growing Outsourcing Trend in the Market
3.2.2. Market restraint analysis
3.2.2.1. Strict Regulations for Global Pharmaceutical Stability and Storage Services Drugs
3.2.2.2. Issues Associated with Stability Chambers
3.3. Cost Structure of Stability and Storage
3.4. Pharmaceutical Stability & Storage Services Analysis Tools
3.4.1. Porter’s Five Forces Analysis
3.4.2. PESTEL Analysis
3.5. COVID-19 Impact Analysis
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook.
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. High R&D Spending of Pharmaceutical & Biotechnology Companies
3.2.1.2. Growing Demand for Outsourcing Stability Testing Due to High Complexity Associated with Drug Stability
3.2.1.3. Growing Demand for Biosimilars
3.2.1.4. Growing Outsourcing Trend in the Market
3.2.2. Market restraint analysis
3.2.2.1. Strict Regulations for Global Pharmaceutical Stability and Storage Services Drugs
3.2.2.2. Issues Associated with Stability Chambers
3.3. Cost Structure of Stability and Storage
3.4. Pharmaceutical Stability & Storage Services Analysis Tools
3.4.1. Porter’s Five Forces Analysis
3.4.2. PESTEL Analysis
3.5. COVID-19 Impact Analysis
Chapter 4. Pharmaceutical Stability & Storage Services Market: Service Estimates & Trend Analysis
4.1. Segment Dashboard
4.2. Global Pharmaceutical Stability & Storage Services Market; Service Movement Analysis
4.3. Global Pharmaceutical Stability & Storage Services Size & Trend Analysis, by Service, 2018 to 2030 (USD Million)
4.4. Stability
4.4.1. Stability market estimates and forecasts 2018 to 2030 (USD Million)
4.4.2. Drug Substance
4.4.2.1. Drug substance market estimates and forecasts 2018 to 2030 (USD Million)
4.4.3. Stability Indicating Method Validation
4.4.3.1. Stability indicating method validation market estimates and forecasts 2018 to 2030 (USD Million)
4.4.4. Accelerated Stability Testing
4.4.4.1. Accelerated stability testing market estimates and forecasts 2018 to 2030 (USD Million)
4.4.5. Photostability Testing
4.4.5.1. Photostability testing market estimates and forecasts 2018 to 2030 (USD Million)
4.4.6. Other Stability Testing Methods
4.4.6.1. Other stability testing methods market estimates and forecasts 2018 to 2030 (USD Million)
4.5. Storage
4.5.1. Storage market estimates and forecasts 2018 to 2030 (USD Million)
4.5.2. Cold
4.5.2.1. Cold market estimates and forecasts 2018 to 2030 (USD Million)
4.5.3. Non-cold
4.5.3.1. Non-cold market estimates and forecasts 2018 to 2030 (USD Million)
4.2. Global Pharmaceutical Stability & Storage Services Market; Service Movement Analysis
4.3. Global Pharmaceutical Stability & Storage Services Size & Trend Analysis, by Service, 2018 to 2030 (USD Million)
4.4. Stability
4.4.1. Stability market estimates and forecasts 2018 to 2030 (USD Million)
4.4.2. Drug Substance
4.4.2.1. Drug substance market estimates and forecasts 2018 to 2030 (USD Million)
4.4.3. Stability Indicating Method Validation
4.4.3.1. Stability indicating method validation market estimates and forecasts 2018 to 2030 (USD Million)
4.4.4. Accelerated Stability Testing
4.4.4.1. Accelerated stability testing market estimates and forecasts 2018 to 2030 (USD Million)
4.4.5. Photostability Testing
4.4.5.1. Photostability testing market estimates and forecasts 2018 to 2030 (USD Million)
4.4.6. Other Stability Testing Methods
4.4.6.1. Other stability testing methods market estimates and forecasts 2018 to 2030 (USD Million)
4.5. Storage
4.5.1. Storage market estimates and forecasts 2018 to 2030 (USD Million)
4.5.2. Cold
4.5.2.1. Cold market estimates and forecasts 2018 to 2030 (USD Million)
4.5.3. Non-cold
4.5.3.1. Non-cold market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 5. Pharmaceutical Stability & Storage Services Market: Molecule Estimates & Trend Analysis
5.1. Segment Dashboard
5.2. Global Pharmaceutical Stability & Storage Services Market; Molecule Movement Analysis
5.3. Global Pharmaceutical Stability & Storage Services Size & Trend Analysis, by Molecule, 2018 to 2030 (USD Million)
5.4. Small Molecule
5.4.1. Small molecule market estimates and forecasts 2018 to 2030 (USD Million)
5.4.2. Research Products
5.4.2.1. Research products market estimates and forecasts 2018 to 2030 (USD Million)
5.4.3. Commercial Products
5.4.3.1. Commercial products market estimates and forecasts 2018 to 2030 (USD Million)
5.5. Large Molecule
5.5.1. Large molecule market estimates and forecasts 2018 to 2030 (USD Million)
5.5.2. Research Products
5.5.2.1. Research products market estimates and forecasts 2018 to 2030 (USD Million)
5.5.3. Commercial Products
5.5.3.1. Commercial products market estimates and forecasts 2018 to 2030 (USD Million)
5.2. Global Pharmaceutical Stability & Storage Services Market; Molecule Movement Analysis
5.3. Global Pharmaceutical Stability & Storage Services Size & Trend Analysis, by Molecule, 2018 to 2030 (USD Million)
5.4. Small Molecule
5.4.1. Small molecule market estimates and forecasts 2018 to 2030 (USD Million)
5.4.2. Research Products
5.4.2.1. Research products market estimates and forecasts 2018 to 2030 (USD Million)
5.4.3. Commercial Products
5.4.3.1. Commercial products market estimates and forecasts 2018 to 2030 (USD Million)
5.5. Large Molecule
5.5.1. Large molecule market estimates and forecasts 2018 to 2030 (USD Million)
5.5.2. Research Products
5.5.2.1. Research products market estimates and forecasts 2018 to 2030 (USD Million)
5.5.3. Commercial Products
5.5.3.1. Commercial products market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 6. Pharmaceutical Stability & Storage Services Market: Regional Estimates & Trend Analysis
6.1. Regional Market Share Analysis, 2024 & 2030
6.2. Regional Market Dashboard
6.3. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
6.4. North America
6.4.1. North America Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.4.2. U.S.
6.4.2.1. Key country dynamics
6.4.2.2. Competitive scenario
6.4.2.3. Regulatory framework
6.4.2.4. U.S. market estimates and forecasts 2018 to 2030 (USD Million)
6.4.3. Canada
6.4.3.1. Key country dynamics
6.4.3.2. Competitive scenario
6.4.3.3. Regulatory framework
6.4.3.4. Canada market estimates and forecasts 2018 to 2030 (USD Million)
6.4.4. Mexico
6.4.4.1. Key country dynamics
6.4.4.2. Competitive scenario
6.4.4.3. Regulatory framework
6.4.4.4. Mexico market estimates and forecasts 2018 to 2030 (USD Million)
6.5. Europe
6.5.1. Europe Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.5.2. UK
6.5.2.1. Key country dynamics
6.5.2.2. Competitive scenario
6.5.2.3. Regulatory framework
6.5.2.4. UK market estimates and forecasts 2018 to 2030 (USD Million)
6.5.3. Germany
6.5.3.1. Key country dynamics
6.5.3.2. Competitive scenario
6.5.3.3. Regulatory framework
6.5.3.4. Germany market estimates and forecasts 2018 to 2030 (USD Million)
6.5.4. France
6.5.4.1. Key country dynamics
6.5.4.2. Competitive scenario
6.5.4.3. Regulatory framework
6.5.4.4. France market estimates and forecasts 2018 to 2030 (USD Million)
6.5.5. Italy
6.5.5.1. Key country dynamics
6.5.5.2. Competitive scenario
6.5.5.3. Regulatory framework
6.5.5.4. Italy market estimates and forecasts 2018 to 2030 (USD Million)
6.5.6. Spain
6.5.6.1. Key country dynamics
6.5.6.2. Competitive scenario
6.5.6.3. Regulatory framework
6.5.6.4. Spain market estimates and forecasts 2018 to 2030 (USD Million)
6.5.7. Denmark
6.5.7.1. Key country dynamics
6.5.7.2. Competitive scenario
6.5.7.3. Regulatory framework
6.5.7.4. Denmark market estimates and forecasts 2018 to 2030 (USD Million)
6.5.8. Sweden
6.5.8.1. Key country dynamics
6.5.8.2. Competitive scenario
6.5.8.3. Regulatory framework
6.5.8.4. Sweden market estimates and forecasts 2018 to 2030 (USD Million)
6.5.9. Norway
6.5.9.1. Key country dynamics
6.5.9.2. Competitive scenario
6.5.9.3. Regulatory framework
6.5.9.4. Norway market estimates and forecasts 2018 to 2030 (USD Million)
6.6. Asia Pacific
6.6.1. Asia Pacific Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.6.2. Japan
6.6.2.1. Key country dynamics
6.6.2.2. Competitive scenario
6.6.2.3. Regulatory framework
6.6.2.4. Japan market estimates and forecasts 2018 to 2030 (USD Million)
6.6.3. China
6.6.3.1. Key country dynamics
6.6.3.2. Competitive scenario
6.6.3.3. Regulatory framework
6.6.3.4. China market estimates and forecasts 2018 to 2030 (USD Million)
6.6.4. India
6.6.4.1. Key country dynamics
6.6.4.2. Competitive scenario
6.6.4.3. Regulatory framework
6.6.4.4. India market estimates and forecasts 2018 to 2030 (USD Million)
6.6.5. Australia
6.6.5.1. Key country dynamics
6.6.5.2. Competitive scenario
6.6.5.3. Regulatory framework
6.6.5.4. Australia market estimates and forecasts 2018 to 2030 (USD Million)
6.6.6. South Korea
6.6.6.1. Key country dynamics
6.6.6.2. Competitive scenario
6.6.6.3. Regulatory framework
6.6.6.4. South Korea market estimates and forecasts 2018 to 2030 (USD Million)
6.6.7. Thailand
6.6.7.1. Key country dynamics
6.6.7.2. Competitive scenario
6.6.7.3. Regulatory framework
6.6.7.4. Thailand market estimates and forecasts 2018 to 2030 (USD Million)
6.7. Latin America
6.7.1. Latin America Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.7.2. Brazil
6.7.2.1. Key country dynamics
6.7.2.2. Competitive scenario
6.7.2.3. Regulatory framework
6.7.2.4. Brazil market estimates and forecasts 2018 to 2030 (USD Million)
6.7.3. Argentina
6.7.3.1. Key country dynamics
6.7.3.2. Competitive scenario
6.7.3.3. Regulatory framework
6.7.3.4. Argentina market estimates and forecasts 2018 to 2030 (USD Million)
6.8. MEA
6.8.1. MEA Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.8.2. South Africa
6.8.2.1. Key country dynamics
6.8.2.2. Competitive scenario
6.8.2.3. Regulatory framework
6.8.2.4. South Africa market estimates and forecasts 2018 to 2030 (USD Million)
6.8.3. Saudi Arabia
6.8.3.1. Key country dynamics
6.8.3.2. Competitive scenario
6.8.3.3. Regulatory framework
6.8.3.4. Saudi Arabia market estimates and forecasts 2018 to 2030 (USD Million)
6.8.4. UAE
6.8.4.1. Key country dynamics
6.8.4.2. Competitive scenario
6.8.4.3. Regulatory framework
6.8.4.4. UAE market estimates and forecasts 2018 to 2030 (USD Million)
6.8.5. Kuwait
6.8.5.1. Key country dynamics
6.8.5.2. Competitive scenario
6.8.5.3. Regulatory framework
6.8.5.4. Kuwait market estimates and forecasts 2018 to 2030 (USD Million)
6.2. Regional Market Dashboard
6.3. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
6.4. North America
6.4.1. North America Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.4.2. U.S.
6.4.2.1. Key country dynamics
6.4.2.2. Competitive scenario
6.4.2.3. Regulatory framework
6.4.2.4. U.S. market estimates and forecasts 2018 to 2030 (USD Million)
6.4.3. Canada
6.4.3.1. Key country dynamics
6.4.3.2. Competitive scenario
6.4.3.3. Regulatory framework
6.4.3.4. Canada market estimates and forecasts 2018 to 2030 (USD Million)
6.4.4. Mexico
6.4.4.1. Key country dynamics
6.4.4.2. Competitive scenario
6.4.4.3. Regulatory framework
6.4.4.4. Mexico market estimates and forecasts 2018 to 2030 (USD Million)
6.5. Europe
6.5.1. Europe Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.5.2. UK
6.5.2.1. Key country dynamics
6.5.2.2. Competitive scenario
6.5.2.3. Regulatory framework
6.5.2.4. UK market estimates and forecasts 2018 to 2030 (USD Million)
6.5.3. Germany
6.5.3.1. Key country dynamics
6.5.3.2. Competitive scenario
6.5.3.3. Regulatory framework
6.5.3.4. Germany market estimates and forecasts 2018 to 2030 (USD Million)
6.5.4. France
6.5.4.1. Key country dynamics
6.5.4.2. Competitive scenario
6.5.4.3. Regulatory framework
6.5.4.4. France market estimates and forecasts 2018 to 2030 (USD Million)
6.5.5. Italy
6.5.5.1. Key country dynamics
6.5.5.2. Competitive scenario
6.5.5.3. Regulatory framework
6.5.5.4. Italy market estimates and forecasts 2018 to 2030 (USD Million)
6.5.6. Spain
6.5.6.1. Key country dynamics
6.5.6.2. Competitive scenario
6.5.6.3. Regulatory framework
6.5.6.4. Spain market estimates and forecasts 2018 to 2030 (USD Million)
6.5.7. Denmark
6.5.7.1. Key country dynamics
6.5.7.2. Competitive scenario
6.5.7.3. Regulatory framework
6.5.7.4. Denmark market estimates and forecasts 2018 to 2030 (USD Million)
6.5.8. Sweden
6.5.8.1. Key country dynamics
6.5.8.2. Competitive scenario
6.5.8.3. Regulatory framework
6.5.8.4. Sweden market estimates and forecasts 2018 to 2030 (USD Million)
6.5.9. Norway
6.5.9.1. Key country dynamics
6.5.9.2. Competitive scenario
6.5.9.3. Regulatory framework
6.5.9.4. Norway market estimates and forecasts 2018 to 2030 (USD Million)
6.6. Asia Pacific
6.6.1. Asia Pacific Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.6.2. Japan
6.6.2.1. Key country dynamics
6.6.2.2. Competitive scenario
6.6.2.3. Regulatory framework
6.6.2.4. Japan market estimates and forecasts 2018 to 2030 (USD Million)
6.6.3. China
6.6.3.1. Key country dynamics
6.6.3.2. Competitive scenario
6.6.3.3. Regulatory framework
6.6.3.4. China market estimates and forecasts 2018 to 2030 (USD Million)
6.6.4. India
6.6.4.1. Key country dynamics
6.6.4.2. Competitive scenario
6.6.4.3. Regulatory framework
6.6.4.4. India market estimates and forecasts 2018 to 2030 (USD Million)
6.6.5. Australia
6.6.5.1. Key country dynamics
6.6.5.2. Competitive scenario
6.6.5.3. Regulatory framework
6.6.5.4. Australia market estimates and forecasts 2018 to 2030 (USD Million)
6.6.6. South Korea
6.6.6.1. Key country dynamics
6.6.6.2. Competitive scenario
6.6.6.3. Regulatory framework
6.6.6.4. South Korea market estimates and forecasts 2018 to 2030 (USD Million)
6.6.7. Thailand
6.6.7.1. Key country dynamics
6.6.7.2. Competitive scenario
6.6.7.3. Regulatory framework
6.6.7.4. Thailand market estimates and forecasts 2018 to 2030 (USD Million)
6.7. Latin America
6.7.1. Latin America Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.7.2. Brazil
6.7.2.1. Key country dynamics
6.7.2.2. Competitive scenario
6.7.2.3. Regulatory framework
6.7.2.4. Brazil market estimates and forecasts 2018 to 2030 (USD Million)
6.7.3. Argentina
6.7.3.1. Key country dynamics
6.7.3.2. Competitive scenario
6.7.3.3. Regulatory framework
6.7.3.4. Argentina market estimates and forecasts 2018 to 2030 (USD Million)
6.8. MEA
6.8.1. MEA Market Estimates and Forecasts 2018 to 2030 (USD Million)
6.8.2. South Africa
6.8.2.1. Key country dynamics
6.8.2.2. Competitive scenario
6.8.2.3. Regulatory framework
6.8.2.4. South Africa market estimates and forecasts 2018 to 2030 (USD Million)
6.8.3. Saudi Arabia
6.8.3.1. Key country dynamics
6.8.3.2. Competitive scenario
6.8.3.3. Regulatory framework
6.8.3.4. Saudi Arabia market estimates and forecasts 2018 to 2030 (USD Million)
6.8.4. UAE
6.8.4.1. Key country dynamics
6.8.4.2. Competitive scenario
6.8.4.3. Regulatory framework
6.8.4.4. UAE market estimates and forecasts 2018 to 2030 (USD Million)
6.8.5. Kuwait
6.8.5.1. Key country dynamics
6.8.5.2. Competitive scenario
6.8.5.3. Regulatory framework
6.8.5.4. Kuwait market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 7. Competitive Landscape
7.1. Company Categorization
7.2. Company Market Position Analysis, 2024
7.3. Company Profiles
7.3.1. Catalent, Inc
7.3.1.1. Company overview
7.3.1.2. Financial performance
7.3.1.3. Service benchmarking
7.3.1.4. Strategic initiatives
7.3.2. Almac Group
7.3.2.1. Company overview
7.3.2.2. Financial performance
7.3.2.3. Service benchmarking
7.3.2.4. Strategic initiatives
7.3.3. Charles River Laboratories
7.3.3.1. Company overview
7.3.3.2. Financial performance
7.3.3.3. Service benchmarking
7.3.3.4. Strategic initiatives
7.3.4. SGS Société Générale de Surveillance SA.
7.3.4.1. Company overview
7.3.4.2. Financial performance
7.3.4.3. Service benchmarking
7.3.4.4. Strategic initiatives
7.3.5. Eurofins Scientific
7.3.5.1. Company overview
7.3.5.2. Financial performance
7.3.5.3. Service benchmarking
7.3.5.4. Strategic initiatives
7.3.6. Intertek Group plc
7.3.6.1. Company overview
7.3.6.2. Financial performance
7.3.6.3. Service benchmarking
7.3.6.4. Strategic initiatives
7.3.7. Lucideon Limited
7.3.7.1. Company overview
7.3.7.2. Financial performance
7.3.7.3. Service benchmarking
7.3.7.4. Strategic initiatives
7.3.8. Alcami Corporation
7.3.8.1. Company overview
7.3.8.2. Financial performance
7.3.8.3. Service benchmarking
7.3.8.4. Strategic initiatives
7.3.9. Element Materials Technology
7.3.9.1. Company overview
7.3.9.2. Financial performance
7.3.9.3. Service benchmarking
7.3.9.4. Strategic initiatives
7.3.10. BioLife Solutions Inc.
7.3.10.1. Company overview
7.3.10.2. Financial performance
7.3.10.3. Service benchmarking
7.3.10.4. Strategic initiatives
7.3.11. Q1 Scientific
7.3.11.1. Company overview
7.3.11.2. Financial performance
7.3.11.3. Service benchmarking
7.3.11.4. Strategic initiatives
7.3.12. Reading Scientific Services Ltd.
7.3.12.1. Company overview
7.3.12.2. Financial performance
7.3.12.3. Service benchmarking
7.3.12.4. Strategic initiatives
7.3.13. Roylance Stability Storage Limited
7.3.13.1. Company overview
7.3.13.2. Financial performance
7.3.13.3. Service benchmarking
7.3.13.4. Strategic initiatives
7.3.14. ALS
7.3.14.1. Company overview
7.3.14.2. Financial performance
7.3.14.3. Service benchmarking
7.3.14.4. Strategic initiatives
7.3.15. Q Laboratories
7.3.15.1. Company overview
7.3.15.2. Financial performance
7.3.15.3. Service benchmarking
7.3.15.4. Strategic initiatives
7.3.16. Auriga Research Private Limited
7.3.16.1. Company overview
7.3.16.2. Financial performance
7.3.16.3. Service benchmarking
7.3.16.4. Strategic initiatives
7.3.17. PD Partners
7.3.17.1. Company overview
7.3.17.2. Financial performance
7.3.17.3. Service benchmarking
7.3.17.4. Strategic initiatives
7.3.18. Precision Stability Storage
7.3.18.1. Company overview
7.3.18.2. Financial performance
7.3.18.3. Service benchmarking
7.3.18.4. Strategic initiatives
7.2. Company Market Position Analysis, 2024
7.3. Company Profiles
7.3.1. Catalent, Inc
7.3.1.1. Company overview
7.3.1.2. Financial performance
7.3.1.3. Service benchmarking
7.3.1.4. Strategic initiatives
7.3.2. Almac Group
7.3.2.1. Company overview
7.3.2.2. Financial performance
7.3.2.3. Service benchmarking
7.3.2.4. Strategic initiatives
7.3.3. Charles River Laboratories
7.3.3.1. Company overview
7.3.3.2. Financial performance
7.3.3.3. Service benchmarking
7.3.3.4. Strategic initiatives
7.3.4. SGS Société Générale de Surveillance SA.
7.3.4.1. Company overview
7.3.4.2. Financial performance
7.3.4.3. Service benchmarking
7.3.4.4. Strategic initiatives
7.3.5. Eurofins Scientific
7.3.5.1. Company overview
7.3.5.2. Financial performance
7.3.5.3. Service benchmarking
7.3.5.4. Strategic initiatives
7.3.6. Intertek Group plc
7.3.6.1. Company overview
7.3.6.2. Financial performance
7.3.6.3. Service benchmarking
7.3.6.4. Strategic initiatives
7.3.7. Lucideon Limited
7.3.7.1. Company overview
7.3.7.2. Financial performance
7.3.7.3. Service benchmarking
7.3.7.4. Strategic initiatives
7.3.8. Alcami Corporation
7.3.8.1. Company overview
7.3.8.2. Financial performance
7.3.8.3. Service benchmarking
7.3.8.4. Strategic initiatives
7.3.9. Element Materials Technology
7.3.9.1. Company overview
7.3.9.2. Financial performance
7.3.9.3. Service benchmarking
7.3.9.4. Strategic initiatives
7.3.10. BioLife Solutions Inc.
7.3.10.1. Company overview
7.3.10.2. Financial performance
7.3.10.3. Service benchmarking
7.3.10.4. Strategic initiatives
7.3.11. Q1 Scientific
7.3.11.1. Company overview
7.3.11.2. Financial performance
7.3.11.3. Service benchmarking
7.3.11.4. Strategic initiatives
7.3.12. Reading Scientific Services Ltd.
7.3.12.1. Company overview
7.3.12.2. Financial performance
7.3.12.3. Service benchmarking
7.3.12.4. Strategic initiatives
7.3.13. Roylance Stability Storage Limited
7.3.13.1. Company overview
7.3.13.2. Financial performance
7.3.13.3. Service benchmarking
7.3.13.4. Strategic initiatives
7.3.14. ALS
7.3.14.1. Company overview
7.3.14.2. Financial performance
7.3.14.3. Service benchmarking
7.3.14.4. Strategic initiatives
7.3.15. Q Laboratories
7.3.15.1. Company overview
7.3.15.2. Financial performance
7.3.15.3. Service benchmarking
7.3.15.4. Strategic initiatives
7.3.16. Auriga Research Private Limited
7.3.16.1. Company overview
7.3.16.2. Financial performance
7.3.16.3. Service benchmarking
7.3.16.4. Strategic initiatives
7.3.17. PD Partners
7.3.17.1. Company overview
7.3.17.2. Financial performance
7.3.17.3. Service benchmarking
7.3.17.4. Strategic initiatives
7.3.18. Precision Stability Storage
7.3.18.1. Company overview
7.3.18.2. Financial performance
7.3.18.3. Service benchmarking
7.3.18.4. Strategic initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviations
Table 3 Global Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 4 Global Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 5 Global Pharmaceutical Stability & Storage Services, by Region, 2018 - 2030 (USD Million)
Table 6 North America Pharmaceutical Stability & Storage Services, by Country, 2018 - 2030 (USD Million)
Table 7 North America Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 8 North America Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 9 U.S. Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 10 U.S. Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 11 Canada Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 12 Canada Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 13 Mexico Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 14 Mexico Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 15 UK Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 16 UK Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 17 Germany Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 18 Germany Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 19 France Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 20 France Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 21 Italy Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 22 Italy Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 23 Spain Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 24 Spain Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 25 Sweden Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 26 Sweden Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 27 Norway Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 28 Norway Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 29 Denmark Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 30 Denmark Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 31 India Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 32 India Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 33 China Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 34 China Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 35 Japan Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 36 Japan Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 37 India Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 38 India Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 39 Australia Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 40 Australia Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 41 Thailand Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 42 Thailand Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 43 South Korea Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 44 South Korea Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 45 Brazil Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 46 Brazil Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 47 Argentina Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 48 Argentina Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 49 Saudi Arabia Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 50 Saudi Arabia Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 51 UAE Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 52 UAE Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 53 Kuwait Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 54 Kuwait Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 2 List of Abbreviations
Table 3 Global Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 4 Global Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 5 Global Pharmaceutical Stability & Storage Services, by Region, 2018 - 2030 (USD Million)
Table 6 North America Pharmaceutical Stability & Storage Services, by Country, 2018 - 2030 (USD Million)
Table 7 North America Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 8 North America Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 9 U.S. Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 10 U.S. Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 11 Canada Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 12 Canada Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 13 Mexico Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 14 Mexico Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 15 UK Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 16 UK Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 17 Germany Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 18 Germany Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 19 France Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 20 France Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 21 Italy Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 22 Italy Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 23 Spain Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 24 Spain Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 25 Sweden Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 26 Sweden Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 27 Norway Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 28 Norway Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 29 Denmark Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 30 Denmark Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 31 India Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 32 India Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 33 China Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 34 China Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 35 Japan Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 36 Japan Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 37 India Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 38 India Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 39 Australia Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 40 Australia Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 41 Thailand Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 42 Thailand Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 43 South Korea Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 44 South Korea Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 45 Brazil Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 46 Brazil Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 47 Argentina Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 48 Argentina Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 49 Saudi Arabia Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 50 Saudi Arabia Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 51 UAE Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 52 UAE Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
Table 53 Kuwait Pharmaceutical Stability & Storage Services, by Service, 2018 - 2030 (USD Million)
Table 54 Kuwait Pharmaceutical Stability & Storage Services, by Molecule, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Information Procurement
Fig. 2 Primary Research Pattern
Fig. 3 Market Research Approaches
Fig. 4 Value Chain-Based Sizing & Forecasting
Fig. 5 Market Formulation & Validation
Fig. 6 Pharmaceutical Stability & Storage Services, Market Segmentation
Fig. 7 Market Driver Relevance Analysis (Current & Future Impact)
Fig. 8 Market Restraint Relevance Analysis (Current & Future Impact)
Fig. 9 Porter’s Five Forces Analysis
Fig. 10 PESTEL Analysis
Fig. 11 Regional Marketplace: Key Takeaways
Fig. 12 Global Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 13 Global Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 14 Global Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 15 Global pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 16 Global Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 17 Global Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 18 Global Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 19 Global Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 20 Global Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 21 Global Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 22 Global Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 23 Global Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 24 Global Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 25 North America Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 26 North America Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 27 North America Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 28 North America Pharmaceutical Stability Services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 29 North America Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 30 North America Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 31 North America Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 32 North America Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 33 North America Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 34 North America Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 35 North America Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 36 North America Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 37 North America Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 38 U.S. Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 39 U.S. Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 40 U.S. Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 41 U.S. pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 42 U.S. Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 43 U.S. Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 44 U.S. Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 45 U.S. Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 46 U.S. Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 47 U.S. Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 48 U.S. Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 49 U.S. Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 50 U.S. Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 51 Canada Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 52 Canada Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 53 Canada Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 54 Canada pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 55 Canada Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 56 Canada Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 57 Canada Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 58 Canada Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 59 Canada Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 60 Canada Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 61 Canada Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 62 Canada Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 63 Canada Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 64 Mexico Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 65 Mexico Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 66 Mexico Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 67 Mexico pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 68 Mexico Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 69 Mexico Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 70 Mexico Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 71 Mexico Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 72 Mexico Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 73 Mexico Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 74 Mexico Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 75 Mexico Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 76 Mexico Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 77 Europe Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 78 Europe Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 79 Europe Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 80 Europe pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 81 Europe Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 82 Europe Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 83 Europe Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 84 Europe Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 85 Europe Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 86 Europe Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 87 Europe Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 88 Europe Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 89 Europe Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 90 UK Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 91 UK Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 92 UK Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 93 UK pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 94 UK Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 95 UK Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 96 UK Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 97 UK Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 98 UK Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 99 UK Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 100 UK Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 101 UK Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 102 UK Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 103 Germany Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 104 Germany Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 105 Germany Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 106 Germany pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 107 Germany Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 108 Germany Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 109 Germany Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 110 Germany Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 111 Germany Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 112 Germany Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 113 Germany Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 114 Germany Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 115 Germany Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 116 France Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 117 France Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 118 France Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 119 France pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 120 France Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 121 France Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 122 France Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 123 France Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 124 France Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 125 France Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 126 France Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 127 France Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 128 France Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 129 Italy Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 130 Italy Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 131 Italy Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 132 Italy pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 133 Italy Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 134 Italy Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 135 Italy Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 136 Italy Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 137 Italy Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 138 Italy Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 139 Italy Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 140 Italy Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 141 Italy Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 142 Spain Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 143 Spain Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 144 Spain Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 145 Spain pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 146 Spain Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 147 Spain Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 148 Spain Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 149 Spain Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 150 Spain Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 151 Spain Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 152 Spain Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 153 Spain Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 154 Spain Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 155 Denmark Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 156 Denmark Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 157 Denmark Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 158 Denmark pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 159 Denmark Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 160 Denmark Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 161 Denmark Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 162 Denmark Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 163 Denmark Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 164 Denmark Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 165 Denmark Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 166 Denmark Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 167 Denmark Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 168 Sweden Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 169 Sweden Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 170 Sweden Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 171 Sweden pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 172 Sweden Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 173 Sweden Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 174 Sweden Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 175 Sweden Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 176 Sweden Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 177 Sweden Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 178 Sweden Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 179 Sweden Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 180 Sweden Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 181 Norway Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 182 Norway Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 183 Norway Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 184 Norway pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 185 Norway Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 186 Norway Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 187 Norway Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 188 Norway Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 189 Norway Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 190 Norway Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 191 Norway Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 192 Norway Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 193 Norway Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 194 Asia Pacific Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 195 Asia Pacific Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 196 Asia Pacific Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 197 Asia Pacific pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 198 Asia Pacific Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 199 Asia Pacific Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 200 Asia Pacific Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 201 Asia Pacific Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 202 Asia Pacific Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 203 Asia Pacific Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 204 Asia Pacific Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 205 Asia Pacific Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 206 Asia Pacific Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 207 Japan Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 208 Japan Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 209 Japan Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 210 Japan pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 211 Japan Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 212 Japan Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 213 Japan Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 214 Japan Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 215 Japan Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 216 Japan Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 217 Japan Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 218 Japan Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 219 Japan Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 220 China Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 221 China Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 222 China Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 223 China pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 224 China Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 225 China Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 226 China Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 227 China Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 228 China Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 229 China Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 230 China Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 231 China Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 232 China Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 233 India Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 234 India Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 235 India Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 236 India pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 237 India Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 238 India Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 239 India Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 240 India Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 241 India Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 242 India Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 243 India Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 244 India Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 245 India Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 246 Australia Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 247 Australia Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 248 Australia Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 249 Australia pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 250 Australia Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 251 Australia Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 252 Australia Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 253 Australia Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 254 Australia Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 255 Australia Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 256 Australia Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 257 Australia Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 258 Australia Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 259 South Korea Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 260 South Korea Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 261 South Korea Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 262 South Korea pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 263 South Korea Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 264 South Korea Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 265 South Korea Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 266 South Korea Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 267 South Korea Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 268 South Korea Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 269 South Korea Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 270 South Korea Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 271 South Korea Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 272 Thailand Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 273 Thailand Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 274 Thailand Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 275 Thailand pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 276 Thailand Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 277 Thailand Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 278 Thailand Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 279 Thailand Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 280 Thailand Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 281 Thailand Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 282 Thailand Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 283 Thailand Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 284 Thailand Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 285 Latin America Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 286 Latin America Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 287 Latin America Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 288 Latin America pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 289 Latin America Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 290 Latin America Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 291 Latin America Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 292 Latin America Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 293 Latin America Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 294 Latin America Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 295 Latin America Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 296 Latin America Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 297 Latin America Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 298 Brazil Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 299 Brazil Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 300 Brazil Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 301 Brazil pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 302 Brazil Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 303 Brazil Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 304 Brazil Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 305 Brazil Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 306 Brazil Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 307 Brazil Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 308 Brazil Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 309 Brazil Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 310 Brazil Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 311 Argentina Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 312 Argentina Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 313 Argentina Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 314 Argentina pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 2 Primary Research Pattern
Fig. 3 Market Research Approaches
Fig. 4 Value Chain-Based Sizing & Forecasting
Fig. 5 Market Formulation & Validation
Fig. 6 Pharmaceutical Stability & Storage Services, Market Segmentation
Fig. 7 Market Driver Relevance Analysis (Current & Future Impact)
Fig. 8 Market Restraint Relevance Analysis (Current & Future Impact)
Fig. 9 Porter’s Five Forces Analysis
Fig. 10 PESTEL Analysis
Fig. 11 Regional Marketplace: Key Takeaways
Fig. 12 Global Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 13 Global Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 14 Global Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 15 Global pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 16 Global Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 17 Global Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 18 Global Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 19 Global Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 20 Global Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 21 Global Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 22 Global Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 23 Global Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 24 Global Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 25 North America Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 26 North America Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 27 North America Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 28 North America Pharmaceutical Stability Services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 29 North America Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 30 North America Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 31 North America Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 32 North America Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 33 North America Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 34 North America Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 35 North America Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 36 North America Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 37 North America Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 38 U.S. Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 39 U.S. Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 40 U.S. Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 41 U.S. pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 42 U.S. Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 43 U.S. Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 44 U.S. Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 45 U.S. Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 46 U.S. Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 47 U.S. Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 48 U.S. Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 49 U.S. Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 50 U.S. Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 51 Canada Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 52 Canada Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 53 Canada Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 54 Canada pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 55 Canada Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 56 Canada Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 57 Canada Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 58 Canada Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 59 Canada Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 60 Canada Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 61 Canada Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 62 Canada Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 63 Canada Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 64 Mexico Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 65 Mexico Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 66 Mexico Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 67 Mexico pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 68 Mexico Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 69 Mexico Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 70 Mexico Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 71 Mexico Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 72 Mexico Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 73 Mexico Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 74 Mexico Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 75 Mexico Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 76 Mexico Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 77 Europe Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 78 Europe Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 79 Europe Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 80 Europe pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 81 Europe Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 82 Europe Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 83 Europe Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 84 Europe Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 85 Europe Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 86 Europe Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 87 Europe Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 88 Europe Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 89 Europe Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 90 UK Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 91 UK Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 92 UK Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 93 UK pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 94 UK Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 95 UK Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 96 UK Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 97 UK Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 98 UK Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 99 UK Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 100 UK Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 101 UK Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 102 UK Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 103 Germany Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 104 Germany Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 105 Germany Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 106 Germany pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 107 Germany Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 108 Germany Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 109 Germany Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 110 Germany Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 111 Germany Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 112 Germany Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 113 Germany Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 114 Germany Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 115 Germany Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 116 France Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 117 France Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 118 France Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 119 France pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 120 France Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 121 France Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 122 France Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 123 France Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 124 France Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 125 France Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 126 France Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 127 France Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 128 France Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 129 Italy Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 130 Italy Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 131 Italy Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 132 Italy pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 133 Italy Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 134 Italy Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 135 Italy Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 136 Italy Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 137 Italy Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 138 Italy Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 139 Italy Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 140 Italy Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 141 Italy Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 142 Spain Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 143 Spain Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 144 Spain Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 145 Spain pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 146 Spain Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 147 Spain Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 148 Spain Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 149 Spain Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 150 Spain Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 151 Spain Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 152 Spain Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 153 Spain Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 154 Spain Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 155 Denmark Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 156 Denmark Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 157 Denmark Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 158 Denmark pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 159 Denmark Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 160 Denmark Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 161 Denmark Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 162 Denmark Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 163 Denmark Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 164 Denmark Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 165 Denmark Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 166 Denmark Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 167 Denmark Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 168 Sweden Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 169 Sweden Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 170 Sweden Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 171 Sweden pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 172 Sweden Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 173 Sweden Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 174 Sweden Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 175 Sweden Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 176 Sweden Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 177 Sweden Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 178 Sweden Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 179 Sweden Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 180 Sweden Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 181 Norway Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 182 Norway Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 183 Norway Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 184 Norway pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 185 Norway Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 186 Norway Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 187 Norway Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 188 Norway Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 189 Norway Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 190 Norway Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 191 Norway Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 192 Norway Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 193 Norway Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 194 Asia Pacific Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 195 Asia Pacific Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 196 Asia Pacific Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 197 Asia Pacific pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 198 Asia Pacific Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 199 Asia Pacific Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 200 Asia Pacific Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 201 Asia Pacific Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 202 Asia Pacific Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 203 Asia Pacific Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 204 Asia Pacific Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 205 Asia Pacific Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 206 Asia Pacific Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 207 Japan Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 208 Japan Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 209 Japan Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 210 Japan pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 211 Japan Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 212 Japan Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 213 Japan Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 214 Japan Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 215 Japan Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 216 Japan Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 217 Japan Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 218 Japan Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 219 Japan Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 220 China Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 221 China Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 222 China Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 223 China pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 224 China Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 225 China Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 226 China Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 227 China Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 228 China Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 229 China Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 230 China Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 231 China Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 232 China Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 233 India Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 234 India Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 235 India Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 236 India pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 237 India Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 238 India Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 239 India Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 240 India Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 241 India Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 242 India Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 243 India Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 244 India Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 245 India Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 246 Australia Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 247 Australia Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 248 Australia Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 249 Australia pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 250 Australia Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 251 Australia Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 252 Australia Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 253 Australia Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 254 Australia Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 255 Australia Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 256 Australia Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 257 Australia Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 258 Australia Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 259 South Korea Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 260 South Korea Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 261 South Korea Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 262 South Korea pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 263 South Korea Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 264 South Korea Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 265 South Korea Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 266 South Korea Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 267 South Korea Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 268 South Korea Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 269 South Korea Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 270 South Korea Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 271 South Korea Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 272 Thailand Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 273 Thailand Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 274 Thailand Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 275 Thailand pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 276 Thailand Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 277 Thailand Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 278 Thailand Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 279 Thailand Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 280 Thailand Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 281 Thailand Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 282 Thailand Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 283 Thailand Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 284 Thailand Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 285 Latin America Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 286 Latin America Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 287 Latin America Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 288 Latin America pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 289 Latin America Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 290 Latin America Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 291 Latin America Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 292 Latin America Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 293 Latin America Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 294 Latin America Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 295 Latin America Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 296 Latin America Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 297 Latin America Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 298 Brazil Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 299 Brazil Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 300 Brazil Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 301 Brazil pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Fig. 302 Brazil Pharmaceutical Stability Services, For Accelerated Stability Testing, 2018 - 2030 (USD Million)
Fig. 303 Brazil Pharmaceutical Stability Services, For Photostability Testing, 2018 - 2030 (USD Million)
Fig. 304 Brazil Pharmaceutical Stability Services, For Other Stability Testing Methods, 2018 - 2030 (USD Million)
Fig. 305 Brazil Pharmaceutical Storage Services, for Cold, 2018 - 2030 (USD Million)
Fig. 306 Brazil Pharmaceutical Storage Services, for Non-cold, 2018 - 2030 (USD Million)
Fig. 307 Brazil Small Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 308 Brazil Small Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 309 Brazil Large Molecule Pharmaceutical Stability & Storage Services, for Research Products, 2018 - 2030 (USD Million)
Fig. 310 Brazil Large Molecule Pharmaceutical Stability & Storage Services, for Commercial Products
Fig. 311 Argentina Pharmaceutical Stability & Storage Services, for Service, 2018 - 2030 (USD Million)
Fig. 312 Argentina Pharmaceutical Stability & Storage Services, for Stability, 2018 - 2030 (USD Million)
Fig. 313 Argentina Pharmaceutical Stability Services, for Drug Substance, 2018 - 2030 (USD Million)
Fig. 314 Argentina pharmaceutical stability services, for Stability Indicating Method Validation, 2018 - 2030 (USD Million)
Companies Mentioned
- Catalent, Inc
- Almac Group
- Charles River Laboratories
- Eurofins Scientific
- Intertek Group plc
- Lucideon Limited
- Alcami Corporation
- Element Materials Technology
- BioLife Solutions Inc.
- Q1 Scientific
- Reading Scientific Services Ltd.
- Roylance Stability Storage Limited
- ALS
- Q Laboratories
- Auriga Research Private Limited
- PD Partners
- Precision Stability Storage
- SGS Société Générale de Surveillance SA.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 130 |
| Published | December 2024 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.92 billion |
| Forecasted Market Value ( USD | $ 2.8 billion |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |