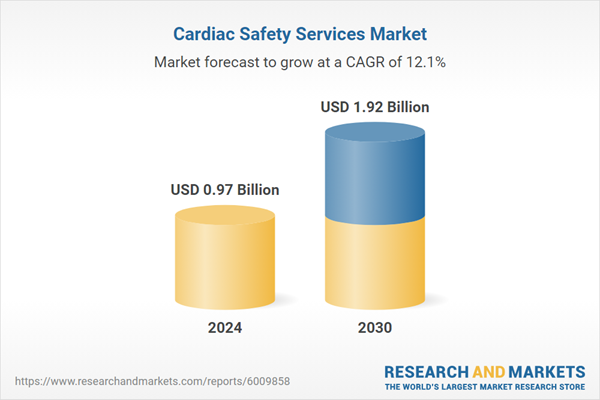
The global cardiac safety services market size is anticipated to reach USD 1.92 billion by 2030 and is projected to grow at a CAGR of 12.1% from 2025 to 2030. This growth is driven by the alarming prevalence of cardiovascular diseases (CVDs) and significant advancements in medical technology. As per the CDC, in U.S., one person dies due to any cardiovascular disease every 33 seconds, underscoring the critical need for effective cardiac monitoring and intervention. In 2022, heart disease caused 702,880 deaths, representing one in every five deaths and highlighting the vast market potential for cardiac safety services.
Moreover, approximately 805,000 people in the U.S. experience a heart attack each year, with 605,000 being first-time incidents and 200,000 involving individuals who have previously suffered a heart attack. Expanding clinical trials, particularly those focused on cardiovascular diseases or involving therapies with potential cardiovascular impacts, drives the demand for cardiac safety services. As pharmaceutical and biopharmaceutical companies invest in developing new cardiovascular drugs and devices, there is an increased need for specialized cardiac safety assessments to ensure that these products do not adversely affect heart health. This growth in clinical trials, combined with the complexity of modern trials, necessitates robust and reliable cardiac safety services to support drug development and regulatory compliance.
Pharmaceutical companies, medical device manufacturers, and academic institutions continually invest in R&D to drive innovation and growth in the cardiac safety services market. Research initiatives often require sophisticated cardiac safety monitoring to support the development of new therapies and technologies. This investment fosters the development of new cardiac safety solutions and supports continuously improving existing services.
This product will be delivered within 2 business days.
Moreover, approximately 805,000 people in the U.S. experience a heart attack each year, with 605,000 being first-time incidents and 200,000 involving individuals who have previously suffered a heart attack. Expanding clinical trials, particularly those focused on cardiovascular diseases or involving therapies with potential cardiovascular impacts, drives the demand for cardiac safety services. As pharmaceutical and biopharmaceutical companies invest in developing new cardiovascular drugs and devices, there is an increased need for specialized cardiac safety assessments to ensure that these products do not adversely affect heart health. This growth in clinical trials, combined with the complexity of modern trials, necessitates robust and reliable cardiac safety services to support drug development and regulatory compliance.
Pharmaceutical companies, medical device manufacturers, and academic institutions continually invest in R&D to drive innovation and growth in the cardiac safety services market. Research initiatives often require sophisticated cardiac safety monitoring to support the development of new therapies and technologies. This investment fosters the development of new cardiac safety solutions and supports continuously improving existing services.
Cardiac Safety Services Market Report Highlights
- Based on service, the ECG/Holter monitors segment led the market with the largest revenue share of 41.0% in 2024. Electrocardiogram (ECG) and Holter monitors are crucial tools for continuous cardiac monitoring, enabling real-time detection of arrhythmias, ischemic episodes, and other cardiovascular conditions
- Based on type, the integrated segment led the market with the largest revenue share of 63.0% in 2024. This prominence is primarily driven by the increasing demand for comprehensive, multi-functional cardiac monitoring solutions that offer enhanced accuracy, efficiency, and patient convenience
- Based on end use, the pharma & biopharma segment led the market with the largest revenue share of 44.0% in 2024
This product will be delivered within 2 business days.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Service
1.2.2. Type
1.2.3. End Use
1.2.4. Regional scope
1.2.5. Estimates and forecasts timeline
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.4.5.1. Data for primary interviews in North America
1.4.5.2. Data for primary interviews in Europe
1.4.5.3. Data for primary interviews in Asia Pacific
1.4.5.4. Data for primary interviews in Latin America
1.4.5.5. Data for Primary interviews in MEA
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
1.2. Segment Definitions
1.2.1. Service
1.2.2. Type
1.2.3. End Use
1.2.4. Regional scope
1.2.5. Estimates and forecasts timeline
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.4.5.1. Data for primary interviews in North America
1.4.5.2. Data for primary interviews in Europe
1.4.5.3. Data for primary interviews in Asia Pacific
1.4.5.4. Data for primary interviews in Latin America
1.4.5.5. Data for Primary interviews in MEA
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.2.1. Service Outlook
2.2.2. Type
2.2.3. End Use
2.2.4. Regional outlook
2.3. Competitive Insights
2.2. Segment Outlook
2.2.1. Service Outlook
2.2.2. Type
2.2.3. End Use
2.2.4. Regional outlook
2.3. Competitive Insights
Chapter 3. Cardiac Safety Services Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Increasing prevalence of cardiovascular diseases
3.2.1.2. Technological advancements
3.2.1.3. Government Initiatives aimed at improving healthcare outcomes
3.2.2. Market restraint analysis
3.2.2.1. High costs
3.2.2.2. Regulatory challenge
3.3. Cardiac Safety Services Market Analysis Tools
3.3.1. Industry Analysis - Porter’s
3.3.1.1. Supplier power
3.3.1.2. Buyer power
3.3.1.3. Substitution threat
3.3.1.4. Threat of new entrant
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Technological landscape
3.3.2.3. Economic landscape
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Increasing prevalence of cardiovascular diseases
3.2.1.2. Technological advancements
3.2.1.3. Government Initiatives aimed at improving healthcare outcomes
3.2.2. Market restraint analysis
3.2.2.1. High costs
3.2.2.2. Regulatory challenge
3.3. Cardiac Safety Services Market Analysis Tools
3.3.1. Industry Analysis - Porter’s
3.3.1.1. Supplier power
3.3.1.2. Buyer power
3.3.1.3. Substitution threat
3.3.1.4. Threat of new entrant
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Technological landscape
3.3.2.3. Economic landscape
Chapter 4. Cardiac Safety Services Market: Service Estimates & Trend Analysis
4.1. Global Cardiac Safety Services Market: Service Dashboard
4.2. Global Cardiac Safety Services Market: Service Movement Analysis
4.3. Global Cardiac Safety Services Market by Service, Revenue
4.4. ECG/ Holter Monitors
4.4.1. ECG/ Holter Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.4.1.1. ECG Patch
4.4.1.1.1. ECG Patch market estimates and forecasts 2018 to 2030 (USD Million)
4.4.1.2. Holter Monitors
4.4.1.3. Holter Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5. Blood Pressure Monitors
4.5.1. Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5.1.1. Aneroid Blood Pressure Monitors
4.5.1.1.1. Aneroid Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5.1.2. Digital Blood Pressure Monitors
4.5.1.2.1. Digital Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5.1.3. Ambulatory Blood Pressure Monitors
4.5.1.3.1. Ambulatory Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.6. Cardiovascular Imaging
4.6.1. Cardiovascular Imaging market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.1. CT
4.6.1.1.1. CT market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.2. MRI
4.6.1.2.1. MRI market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.3. Ultrasound
4.6.1.3.1. Ultrasound market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.4. Nuclear Medicine
4.6.1.4.1. Nuclear Medicine market estimates and forecasts 2018 to 2030 (USD Million)
4.7. Others
4.7.1. Others market estimates and forecasts 2018 to 2030 (USD Million)
4.2. Global Cardiac Safety Services Market: Service Movement Analysis
4.3. Global Cardiac Safety Services Market by Service, Revenue
4.4. ECG/ Holter Monitors
4.4.1. ECG/ Holter Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.4.1.1. ECG Patch
4.4.1.1.1. ECG Patch market estimates and forecasts 2018 to 2030 (USD Million)
4.4.1.2. Holter Monitors
4.4.1.3. Holter Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5. Blood Pressure Monitors
4.5.1. Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5.1.1. Aneroid Blood Pressure Monitors
4.5.1.1.1. Aneroid Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5.1.2. Digital Blood Pressure Monitors
4.5.1.2.1. Digital Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.5.1.3. Ambulatory Blood Pressure Monitors
4.5.1.3.1. Ambulatory Blood Pressure Monitors market estimates and forecasts 2018 to 2030 (USD Million)
4.6. Cardiovascular Imaging
4.6.1. Cardiovascular Imaging market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.1. CT
4.6.1.1.1. CT market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.2. MRI
4.6.1.2.1. MRI market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.3. Ultrasound
4.6.1.3.1. Ultrasound market estimates and forecasts 2018 to 2030 (USD Million)
4.6.1.4. Nuclear Medicine
4.6.1.4.1. Nuclear Medicine market estimates and forecasts 2018 to 2030 (USD Million)
4.7. Others
4.7.1. Others market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 5. Cardiac Safety Services Market: Type Estimates & Trend Analysis
5.1. Global Cardiac Safety Services Market: Type Dashboard
5.2. Global Cardiac Safety Services Market: Type Movement Analysis
5.3. Global Cardiac Safety Services Market Estimates and Forecasts, By Type, Revenue (USD Million)
5.4. Integrated
5.4.1. Integrated market estimates and forecasts 2018 to 2030 (USD Million)
5.5. Standalone
5.5.1. Standalone market estimates and forecasts 2018 to 2030 (USD Million)
5.2. Global Cardiac Safety Services Market: Type Movement Analysis
5.3. Global Cardiac Safety Services Market Estimates and Forecasts, By Type, Revenue (USD Million)
5.4. Integrated
5.4.1. Integrated market estimates and forecasts 2018 to 2030 (USD Million)
5.5. Standalone
5.5.1. Standalone market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 6. Cardiac Safety Services Market: End Use Estimates & Trend Analysis
6.1. Global Cardiac Safety Services Market: End Use Dashboard
6.2. Global Cardiac Safety Services Market: End Use Movement Analysis
6.3. Global Cardiac Safety Services Market Estimates and Forecasts, By End Use, Revenue (USD Million)
6.4. Pharma & Biopharma Companies
6.4.1. Pharma & Biopharma Companies market estimates and forecasts 2018 to 2030 (USD Million)
6.5. CROs
6.5.1. CROs market estimates and forecasts 2018 to 2030 (USD Million)
6.6. Other
6.6.1. Other market estimates and forecasts 2018 to 2030 (USD Million)
6.2. Global Cardiac Safety Services Market: End Use Movement Analysis
6.3. Global Cardiac Safety Services Market Estimates and Forecasts, By End Use, Revenue (USD Million)
6.4. Pharma & Biopharma Companies
6.4.1. Pharma & Biopharma Companies market estimates and forecasts 2018 to 2030 (USD Million)
6.5. CROs
6.5.1. CROs market estimates and forecasts 2018 to 2030 (USD Million)
6.6. Other
6.6.1. Other market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 7. Cardiac Safety Services Market: Regional Estimates & Trend Analysis by Treatment, Type, and End Use
7.1. Regional Dashboard
7.2. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
7.3. North America
7.3.1. U.S.
7.3.1.1. Key country dynamics
7.3.1.2. Regulatory framework/ reimbursement structure
7.3.1.3. Competitive scenario
7.3.1.4. U.S. market estimates and forecasts 2018 to 2030 (USD Million)
7.3.2. Canada
7.3.2.1. Key country dynamics
7.3.2.2. Regulatory framework/ reimbursement structure
7.3.2.3. Competitive scenario
7.3.2.4. Canada market estimates and forecasts 2018 to 2030 (USD Million)
7.3.3. Mexico
7.3.3.1. Key country dynamics
7.3.3.2. Regulatory framework/ reimbursement structure
7.3.3.3. Competitive scenario
7.3.3.4. Mexico market estimates and forecasts 2018 to 2030 (USD Million)
7.4. Europe
7.4.1. UK
7.4.1.1. Key country dynamics
7.4.1.2. Regulatory framework/ reimbursement structure
7.4.1.3. Competitive scenario
7.4.1.4. UK market estimates and forecasts 2018 to 2030 (USD Million)
7.4.2. Germany
7.4.2.1. Key country dynamics
7.4.2.2. Regulatory framework/ reimbursement structure
7.4.2.3. Competitive scenario
7.4.2.4. Germany market estimates and forecasts 2018 to 2030 (USD Million)
7.4.3. France
7.4.3.1. Key country dynamics
7.4.3.2. Regulatory framework/ reimbursement structure
7.4.3.3. Competitive scenario
7.4.3.4. France market estimates and forecasts 2018 to 2030 (USD Million)
7.4.4. Italy
7.4.4.1. Key country dynamics
7.4.4.2. Regulatory framework/ reimbursement structure
7.4.4.3. Competitive scenario
7.4.4.4. Italy market estimates and forecasts 2018 to 2030 (USD Million)
7.4.5. Spain
7.4.5.1. Key country dynamics
7.4.5.2. Regulatory framework/ reimbursement structure
7.4.5.3. Competitive scenario
7.4.5.4. Spain market estimates and forecasts 2018 to 2030 (USD Million)
7.4.6. Norway
7.4.6.1. Key country dynamics
7.4.6.2. Regulatory framework/ reimbursement structure
7.4.6.3. Competitive scenario
7.4.6.4. Norway market estimates and forecasts 2018 to 2030 (USD Million)
7.4.7. Sweden
7.4.7.1. Key country dynamics
7.4.7.2. Regulatory framework/ reimbursement structure
7.4.7.3. Competitive scenario
7.4.7.4. Sweden market estimates and forecasts 2018 to 2030 (USD Million)
7.4.8. Denmark
7.4.8.1. Key country dynamics
7.4.8.2. Regulatory framework/ reimbursement structure
7.4.8.3. Competitive scenario
7.4.8.4. Denmark market estimates and forecasts 2018 to 2030 (USD Million)
7.5. Asia Pacific
7.5.1. Japan
7.5.1.1. Key country dynamics
7.5.1.2. Regulatory framework/ reimbursement structure
7.5.1.3. Competitive scenario
7.5.1.4. Japan market estimates and forecasts 2018 to 2030 (USD Million)
7.5.2. China
7.5.2.1. Key country dynamics
7.5.2.2. Regulatory framework/ reimbursement structure
7.5.2.3. Competitive scenario
7.5.2.4. China market estimates and forecasts 2018 to 2030 (USD Million)
7.5.3. India
7.5.3.1. Key country dynamics
7.5.3.2. Regulatory framework/ reimbursement structure
7.5.3.3. Competitive scenario
7.5.3.4. India market estimates and forecasts 2018 to 2030 (USD Million)
7.5.4. Australia
7.5.4.1. Key country dynamics
7.5.4.2. Regulatory framework/ reimbursement structure
7.5.4.3. Competitive scenario
7.5.4.4. Australia market estimates and forecasts 2018 to 2030 (USD Million)
7.5.5. South Korea
7.5.5.1. Key country dynamics
7.5.5.2. Regulatory framework/ reimbursement structure
7.5.5.3. Competitive scenario
7.5.5.4. South Korea market estimates and forecasts 2018 to 2030 (USD Million)
7.5.6. Thailand
7.5.6.1. Key country dynamics
7.5.6.2. Regulatory framework/ reimbursement structure
7.5.6.3. Competitive scenario
7.5.6.4. Thailand market estimates and forecasts 2018 to 2030 (USD Million)
7.6. Latin America
7.6.1. Brazil
7.6.1.1. Key country dynamics
7.6.1.2. Regulatory framework/ reimbursement structure
7.6.1.3. Competitive scenario
7.6.1.4. Brazil market estimates and forecasts 2018 to 2030 (USD Million)
7.6.2. Argentina
7.6.2.1. Key country dynamics
7.6.2.2. Regulatory framework/ reimbursement structure
7.6.2.3. Competitive scenario
7.6.2.4. Argentina market estimates and forecasts 2018 to 2030 (USD Million)
7.7. MEA
7.7.1. South Africa
7.7.1.1. Key country dynamics
7.7.1.2. Regulatory framework/ reimbursement structure
7.7.1.3. Competitive scenario
7.7.1.4. South Africa market estimates and forecasts 2018 to 2030 (USD Million)
7.7.2. Saudi Arabia
7.7.2.1. Key country dynamics
7.7.2.2. Regulatory framework/ reimbursement structure
7.7.2.3. Competitive scenario
7.7.2.4. Saudi Arabia market estimates and forecasts 2018 to 2030 (USD Million)
7.7.3. UAE
7.7.3.1. Key country dynamics
7.7.3.2. Regulatory framework/ reimbursement structure
7.7.3.3. Competitive scenario
7.7.3.4. UAE market estimates and forecasts 2018 to 2030 (USD Million)
7.7.4. Kuwait
7.7.4.1. Key country dynamics
7.7.4.2. Regulatory framework/ reimbursement structure
7.7.4.3. Competitive scenario
7.7.4.4. Kuwait market estimates and forecasts 2018 to 2030 (USD Million)
7.2. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
7.3. North America
7.3.1. U.S.
7.3.1.1. Key country dynamics
7.3.1.2. Regulatory framework/ reimbursement structure
7.3.1.3. Competitive scenario
7.3.1.4. U.S. market estimates and forecasts 2018 to 2030 (USD Million)
7.3.2. Canada
7.3.2.1. Key country dynamics
7.3.2.2. Regulatory framework/ reimbursement structure
7.3.2.3. Competitive scenario
7.3.2.4. Canada market estimates and forecasts 2018 to 2030 (USD Million)
7.3.3. Mexico
7.3.3.1. Key country dynamics
7.3.3.2. Regulatory framework/ reimbursement structure
7.3.3.3. Competitive scenario
7.3.3.4. Mexico market estimates and forecasts 2018 to 2030 (USD Million)
7.4. Europe
7.4.1. UK
7.4.1.1. Key country dynamics
7.4.1.2. Regulatory framework/ reimbursement structure
7.4.1.3. Competitive scenario
7.4.1.4. UK market estimates and forecasts 2018 to 2030 (USD Million)
7.4.2. Germany
7.4.2.1. Key country dynamics
7.4.2.2. Regulatory framework/ reimbursement structure
7.4.2.3. Competitive scenario
7.4.2.4. Germany market estimates and forecasts 2018 to 2030 (USD Million)
7.4.3. France
7.4.3.1. Key country dynamics
7.4.3.2. Regulatory framework/ reimbursement structure
7.4.3.3. Competitive scenario
7.4.3.4. France market estimates and forecasts 2018 to 2030 (USD Million)
7.4.4. Italy
7.4.4.1. Key country dynamics
7.4.4.2. Regulatory framework/ reimbursement structure
7.4.4.3. Competitive scenario
7.4.4.4. Italy market estimates and forecasts 2018 to 2030 (USD Million)
7.4.5. Spain
7.4.5.1. Key country dynamics
7.4.5.2. Regulatory framework/ reimbursement structure
7.4.5.3. Competitive scenario
7.4.5.4. Spain market estimates and forecasts 2018 to 2030 (USD Million)
7.4.6. Norway
7.4.6.1. Key country dynamics
7.4.6.2. Regulatory framework/ reimbursement structure
7.4.6.3. Competitive scenario
7.4.6.4. Norway market estimates and forecasts 2018 to 2030 (USD Million)
7.4.7. Sweden
7.4.7.1. Key country dynamics
7.4.7.2. Regulatory framework/ reimbursement structure
7.4.7.3. Competitive scenario
7.4.7.4. Sweden market estimates and forecasts 2018 to 2030 (USD Million)
7.4.8. Denmark
7.4.8.1. Key country dynamics
7.4.8.2. Regulatory framework/ reimbursement structure
7.4.8.3. Competitive scenario
7.4.8.4. Denmark market estimates and forecasts 2018 to 2030 (USD Million)
7.5. Asia Pacific
7.5.1. Japan
7.5.1.1. Key country dynamics
7.5.1.2. Regulatory framework/ reimbursement structure
7.5.1.3. Competitive scenario
7.5.1.4. Japan market estimates and forecasts 2018 to 2030 (USD Million)
7.5.2. China
7.5.2.1. Key country dynamics
7.5.2.2. Regulatory framework/ reimbursement structure
7.5.2.3. Competitive scenario
7.5.2.4. China market estimates and forecasts 2018 to 2030 (USD Million)
7.5.3. India
7.5.3.1. Key country dynamics
7.5.3.2. Regulatory framework/ reimbursement structure
7.5.3.3. Competitive scenario
7.5.3.4. India market estimates and forecasts 2018 to 2030 (USD Million)
7.5.4. Australia
7.5.4.1. Key country dynamics
7.5.4.2. Regulatory framework/ reimbursement structure
7.5.4.3. Competitive scenario
7.5.4.4. Australia market estimates and forecasts 2018 to 2030 (USD Million)
7.5.5. South Korea
7.5.5.1. Key country dynamics
7.5.5.2. Regulatory framework/ reimbursement structure
7.5.5.3. Competitive scenario
7.5.5.4. South Korea market estimates and forecasts 2018 to 2030 (USD Million)
7.5.6. Thailand
7.5.6.1. Key country dynamics
7.5.6.2. Regulatory framework/ reimbursement structure
7.5.6.3. Competitive scenario
7.5.6.4. Thailand market estimates and forecasts 2018 to 2030 (USD Million)
7.6. Latin America
7.6.1. Brazil
7.6.1.1. Key country dynamics
7.6.1.2. Regulatory framework/ reimbursement structure
7.6.1.3. Competitive scenario
7.6.1.4. Brazil market estimates and forecasts 2018 to 2030 (USD Million)
7.6.2. Argentina
7.6.2.1. Key country dynamics
7.6.2.2. Regulatory framework/ reimbursement structure
7.6.2.3. Competitive scenario
7.6.2.4. Argentina market estimates and forecasts 2018 to 2030 (USD Million)
7.7. MEA
7.7.1. South Africa
7.7.1.1. Key country dynamics
7.7.1.2. Regulatory framework/ reimbursement structure
7.7.1.3. Competitive scenario
7.7.1.4. South Africa market estimates and forecasts 2018 to 2030 (USD Million)
7.7.2. Saudi Arabia
7.7.2.1. Key country dynamics
7.7.2.2. Regulatory framework/ reimbursement structure
7.7.2.3. Competitive scenario
7.7.2.4. Saudi Arabia market estimates and forecasts 2018 to 2030 (USD Million)
7.7.3. UAE
7.7.3.1. Key country dynamics
7.7.3.2. Regulatory framework/ reimbursement structure
7.7.3.3. Competitive scenario
7.7.3.4. UAE market estimates and forecasts 2018 to 2030 (USD Million)
7.7.4. Kuwait
7.7.4.1. Key country dynamics
7.7.4.2. Regulatory framework/ reimbursement structure
7.7.4.3. Competitive scenario
7.7.4.4. Kuwait market estimates and forecasts 2018 to 2030 (USD Million)
Chapter 8. Competitive Landscape
8.1. Company/Competition Categorization
8.2. Vendor Landscape
8.2.1. List of key distributors and channel partners
8.2.2. Key company market share analysis, 2023
8.2.3. Medpace
8.2.3.1. Company overview
8.2.3.2. Financial performance
8.2.3.3. Service benchmarking
8.2.3.4. Strategic initiatives
8.2.4. IQVIA
8.2.4.1. Company overview
8.2.4.2. Financial performance
8.2.4.3. Service benchmarking
8.2.4.4. Strategic initiatives
8.2.5. PPD Inc.
8.2.5.1. Company overview
8.2.5.2. Financial performance
8.2.5.3. Service benchmarking
8.2.5.4. Strategic initiatives
8.2.6. Charles River Laboratories
8.2.6.1. Company overview
8.2.6.2. Financial performance
8.2.6.3. Service benchmarking
8.2.6.4. Strategic initiatives
8.2.7. Wuxi AppTec
8.2.7.1. Company overview
8.2.7.2. Financial performance
8.2.7.3. Service benchmarking
8.2.7.4. Strategic initiatives
8.2.8. Eurofins Scientific
8.2.8.1. Company overview
8.2.8.2. Financial performance
8.2.8.3. Service benchmarking
8.2.8.4. Strategic initiatives
8.2.9. Celerion
8.2.9.1. Company overview
8.2.9.2. Financial performance
8.2.9.3. Service benchmarking
8.2.9.4. Strategic initiatives
8.2.10. Nova Research Laboratories
8.2.10.1. Company overview
8.2.10.2. Financial performance
8.2.10.3. Service benchmarking
8.2.10.4. Strategic initiatives
8.2.11. Laboratory Corporation of America Holdings
8.2.11.1. Company overview
8.2.11.2. Financial performance
8.2.11.3. Service benchmarking
8.2.11.4. Strategic initiatives
8.2.12. Koninklije Philips N.V.
8.2.12.1. Company overview
8.2.12.2. Financial performance
8.2.12.3. Service benchmarking
8.2.12.4. Strategic initiatives
8.2.13. ICON Plc.
8.2.13.1. Company overview
8.2.13.2. Financial performance
8.2.13.3. Service benchmarking
8.2.13.4. Strategic initiatives
8.2.14. SGS S.A.
8.2.14.1. Company overview
8.2.14.2. Financial performance
8.2.14.3. Service benchmarking
8.2.14.4. Strategic initiatives
8.2.15. Clario
8.2.15.1. Company overview
8.2.15.2. Financial performance
8.2.15.3. Service benchmarking
8.2.15.4. Strategic initiatives
8.2.16. Certara
8.2.16.1. Company overview
8.2.16.2. Financial performance
8.2.16.3. Service benchmarking
8.2.16.4. Strategic initiatives
8.2. Vendor Landscape
8.2.1. List of key distributors and channel partners
8.2.2. Key company market share analysis, 2023
8.2.3. Medpace
8.2.3.1. Company overview
8.2.3.2. Financial performance
8.2.3.3. Service benchmarking
8.2.3.4. Strategic initiatives
8.2.4. IQVIA
8.2.4.1. Company overview
8.2.4.2. Financial performance
8.2.4.3. Service benchmarking
8.2.4.4. Strategic initiatives
8.2.5. PPD Inc.
8.2.5.1. Company overview
8.2.5.2. Financial performance
8.2.5.3. Service benchmarking
8.2.5.4. Strategic initiatives
8.2.6. Charles River Laboratories
8.2.6.1. Company overview
8.2.6.2. Financial performance
8.2.6.3. Service benchmarking
8.2.6.4. Strategic initiatives
8.2.7. Wuxi AppTec
8.2.7.1. Company overview
8.2.7.2. Financial performance
8.2.7.3. Service benchmarking
8.2.7.4. Strategic initiatives
8.2.8. Eurofins Scientific
8.2.8.1. Company overview
8.2.8.2. Financial performance
8.2.8.3. Service benchmarking
8.2.8.4. Strategic initiatives
8.2.9. Celerion
8.2.9.1. Company overview
8.2.9.2. Financial performance
8.2.9.3. Service benchmarking
8.2.9.4. Strategic initiatives
8.2.10. Nova Research Laboratories
8.2.10.1. Company overview
8.2.10.2. Financial performance
8.2.10.3. Service benchmarking
8.2.10.4. Strategic initiatives
8.2.11. Laboratory Corporation of America Holdings
8.2.11.1. Company overview
8.2.11.2. Financial performance
8.2.11.3. Service benchmarking
8.2.11.4. Strategic initiatives
8.2.12. Koninklije Philips N.V.
8.2.12.1. Company overview
8.2.12.2. Financial performance
8.2.12.3. Service benchmarking
8.2.12.4. Strategic initiatives
8.2.13. ICON Plc.
8.2.13.1. Company overview
8.2.13.2. Financial performance
8.2.13.3. Service benchmarking
8.2.13.4. Strategic initiatives
8.2.14. SGS S.A.
8.2.14.1. Company overview
8.2.14.2. Financial performance
8.2.14.3. Service benchmarking
8.2.14.4. Strategic initiatives
8.2.15. Clario
8.2.15.1. Company overview
8.2.15.2. Financial performance
8.2.15.3. Service benchmarking
8.2.15.4. Strategic initiatives
8.2.16. Certara
8.2.16.1. Company overview
8.2.16.2. Financial performance
8.2.16.3. Service benchmarking
8.2.16.4. Strategic initiatives
List of Tables
Table 1. List Of Abbreviation
Table 2. North America Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 3. North America Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 4. North America Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 5. North America Cardiac Safety Services Market, End Use, 2018 - 2030 (USD Million)
Table 6. U.S. Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 7. U.S. Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 8. U.S. Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 9. Canada Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 10. Canada Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 11. Canada Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 12. Mexico Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 13. Mexico Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 14. Mexico Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 15. Europe Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 16. Europe Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 17. Europe Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 18. Europe Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 19. Germany Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 20. Germany Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 21. Germany Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 22. UK Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 23. UK Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 24. UK Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 25. France Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 26. France Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 27. France Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 28. Italy Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 29. Italy Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 30. Italy Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 31. Spain Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 32. Spain Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 33. Spain Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 34. Denmark Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 35. Denmark Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 36. Denmark Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 37. Sweden Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 38. Sweden Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 39. Sweden Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 40. Norway Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 41. Norway Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 42. Norway Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 43. Asia Pacific Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 44. Asia Pacific Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 45. Asia Pacific Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 46. Asia Pacific Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 47. China Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 48. China Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 49. China Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 50. Japan Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 51. Japan Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 52. Japan Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 53. India Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 54. India Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 55. India Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 56. South Korea Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 57. South Korea Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 58. South Korea Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 59. Australia Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 60. Australia Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 61. Australia Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 62. Thailand Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 63. Thailand Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 64. Thailand Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 65. Latin America Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 66. Latin America Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 67. Latin America Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 68. Latin America Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 69. Brazil Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 70. Brazil Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 71. Brazil Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 72. Argentina Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 73. Argentina Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 74. Argentina Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 75. MEA Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 76. MEA Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 77. MEA Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 78. MEA Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 79. South Africa Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 80. South Africa Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 81. South Africa Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 82. Saudi Arabia Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 83. Saudi Arabia Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 84. Saudi Arabia Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 85. UAE Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 86. UAE Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 87. UAE Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 88. Kuwait Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 89. Kuwait Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 90. Kuwait Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 2. North America Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 3. North America Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 4. North America Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 5. North America Cardiac Safety Services Market, End Use, 2018 - 2030 (USD Million)
Table 6. U.S. Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 7. U.S. Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 8. U.S. Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 9. Canada Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 10. Canada Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 11. Canada Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 12. Mexico Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 13. Mexico Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 14. Mexico Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 15. Europe Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 16. Europe Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 17. Europe Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 18. Europe Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 19. Germany Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 20. Germany Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 21. Germany Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 22. UK Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 23. UK Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 24. UK Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 25. France Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 26. France Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 27. France Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 28. Italy Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 29. Italy Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 30. Italy Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 31. Spain Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 32. Spain Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 33. Spain Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 34. Denmark Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 35. Denmark Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 36. Denmark Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 37. Sweden Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 38. Sweden Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 39. Sweden Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 40. Norway Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 41. Norway Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 42. Norway Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 43. Asia Pacific Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 44. Asia Pacific Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 45. Asia Pacific Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 46. Asia Pacific Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 47. China Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 48. China Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 49. China Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 50. Japan Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 51. Japan Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 52. Japan Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 53. India Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 54. India Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 55. India Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 56. South Korea Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 57. South Korea Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 58. South Korea Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 59. Australia Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 60. Australia Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 61. Australia Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 62. Thailand Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 63. Thailand Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 64. Thailand Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 65. Latin America Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 66. Latin America Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 67. Latin America Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 68. Latin America Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 69. Brazil Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 70. Brazil Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 71. Brazil Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 72. Argentina Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 73. Argentina Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 74. Argentina Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 75. MEA Cardiac Safety Services Market, By Region, 2018 - 2030 (USD Million)
Table 76. MEA Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 77. MEA Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 78. MEA Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 79. South Africa Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 80. South Africa Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 81. South Africa Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 82. Saudi Arabia Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 83. Saudi Arabia Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 84. Saudi Arabia Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 85. UAE Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 86. UAE Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 87. UAE Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
Table 88. Kuwait Cardiac Safety Services Market, By Service, 2018 - 2030 (USD Million)
Table 89. Kuwait Cardiac Safety Services Market, By Type, 2018 - 2030 (USD Million)
Table 90. Kuwait Cardiac Safety Services Market, By End Use, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Market research process
Fig. 2 Data triangulation techniques
Fig. 3 Primary research pattern
Fig. 4 Primary interviews in North America
Fig. 5 Primary interviews in Europe
Fig. 6 Primary interviews in APAC
Fig. 7 Primary interviews in Latin America
Fig. 8 Primary interviews in MEA
Fig. 9 Market research approaches
Fig. 10 Value-chain-based sizing & forecasting
Fig. 11 QFD modeling for market share assessment
Fig. 12 Market formulation & validation
Fig. 13 Cardiac Safety Services Market: Market Outlook
Fig. 14 Cardiac Safety Services Competitive Insights
Fig. 15 Parent market outlook
Fig. 16 Related/ancillary market outlook
Fig. 17 Cardiac Safety Services Market Driver Impact
Fig. 18 Cardiac Safety Services Market Restraint Impact
Fig. 19 Cardiac Safety Services Market Strategic Initiatives Analysis
Fig. 20 Cardiac Safety Services Market: Service Movement Analysis
Fig. 21 Cardiac Safety Services Market: Service Outlook And Key Takeaways
Fig. 22 ECG/Holter Monitors market estimates and forecast, 2018 - 2030
Fig. 23 Blood Pressure Monitors market estimates and forecast, 2018 - 2030
Fig. 24 Cardiovascular Imaging market estimates and forecast, 2018 - 2030
Fig. 25 Others market estimates and forecast, 2018 - 2030
Fig. 26 Cardiac Safety Services Market: Type Movement Analysis
Fig. 27 Cardiac Safety Services Market: Type Outlook And Key Takeaways
Fig. 28 Integrated market estimates and forecast, 2018 - 2030
Fig. 29 Standalone market estimates and forecast, 2018 - 2030
Fig. 30 Cardiac Safety Services Market: End Use Movement Analysis
Fig. 31 Cardiac Safety Services Market: End Use Outlook And Key Takeaways
Fig. 32 Pharma & Biopharma Companies market estimates and forecast, 2018 - 2030
Fig. 33 CROs market estimates and forecast, 2018 - 2030
Fig. 34 Other market estimates and forecast, 2018 - 2030
Fig. 35 Global Cardiac Safety Services Market: Regional Movement Analysis
Fig. 36 Global Cardiac Safety Services Market: Regional Outlook And Key Takeaways
Fig. 37 Global Cardiac Safety Services market share and leading players
Fig. 38 North America, by country
Fig. 39 North America market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 40 US key country dynamics
Fig. 41 US market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 42 Canada key country dynamics
Fig. 43 Canada market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 44 Mexico key country dynamics
Fig. 45 Mexico market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 46 Europe market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 47 UK key country dynamics
Fig. 48 UK market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 49 Germany key country dynamics
Fig. 50 Germany market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 51 France key country dynamics
Fig. 52 France market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 53 Italy key country dynamics
Fig. 54 Italy market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 55 Spain key country dynamics
Fig. 56 Spain market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 57 Denmark key country dynamics
Fig. 58 Denmark market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 59 Sweden key country dynamics
Fig. 60 Sweden market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 61 Norway key country dynamics
Fig. 62 Norway market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 63 Asia Pacific market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 64 China key country dynamics
Fig. 65 China market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 66 Japan key country dynamics
Fig. 67 Japan market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 68 India key country dynamics
Fig. 69 India market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 70 Thailand key country dynamics
Fig. 71 Thailand market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 72 South Korea key country dynamics
Fig. 73 South Korea market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 74 Australia key country dynamics
Fig. 75 Australia market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 76 Latin America market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 77 Brazil key country dynamics
Fig. 78 Brazil market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 79 Argentina key country dynamics
Fig. 80 Argentina market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 81 Middle East and Africa market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 82 South Africa key country dynamics
Fig. 83 South Africa market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 84 Saudi Arabia key country dynamics
Fig. 85 Saudi Arabia market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 86 UAE key country dynamics
Fig. 87 UAE market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 88 Kuwait key country dynamics
Fig. 89 Kuwait market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 90 Market share of key market players - Cardiac Safety Services market
Fig. 2 Data triangulation techniques
Fig. 3 Primary research pattern
Fig. 4 Primary interviews in North America
Fig. 5 Primary interviews in Europe
Fig. 6 Primary interviews in APAC
Fig. 7 Primary interviews in Latin America
Fig. 8 Primary interviews in MEA
Fig. 9 Market research approaches
Fig. 10 Value-chain-based sizing & forecasting
Fig. 11 QFD modeling for market share assessment
Fig. 12 Market formulation & validation
Fig. 13 Cardiac Safety Services Market: Market Outlook
Fig. 14 Cardiac Safety Services Competitive Insights
Fig. 15 Parent market outlook
Fig. 16 Related/ancillary market outlook
Fig. 17 Cardiac Safety Services Market Driver Impact
Fig. 18 Cardiac Safety Services Market Restraint Impact
Fig. 19 Cardiac Safety Services Market Strategic Initiatives Analysis
Fig. 20 Cardiac Safety Services Market: Service Movement Analysis
Fig. 21 Cardiac Safety Services Market: Service Outlook And Key Takeaways
Fig. 22 ECG/Holter Monitors market estimates and forecast, 2018 - 2030
Fig. 23 Blood Pressure Monitors market estimates and forecast, 2018 - 2030
Fig. 24 Cardiovascular Imaging market estimates and forecast, 2018 - 2030
Fig. 25 Others market estimates and forecast, 2018 - 2030
Fig. 26 Cardiac Safety Services Market: Type Movement Analysis
Fig. 27 Cardiac Safety Services Market: Type Outlook And Key Takeaways
Fig. 28 Integrated market estimates and forecast, 2018 - 2030
Fig. 29 Standalone market estimates and forecast, 2018 - 2030
Fig. 30 Cardiac Safety Services Market: End Use Movement Analysis
Fig. 31 Cardiac Safety Services Market: End Use Outlook And Key Takeaways
Fig. 32 Pharma & Biopharma Companies market estimates and forecast, 2018 - 2030
Fig. 33 CROs market estimates and forecast, 2018 - 2030
Fig. 34 Other market estimates and forecast, 2018 - 2030
Fig. 35 Global Cardiac Safety Services Market: Regional Movement Analysis
Fig. 36 Global Cardiac Safety Services Market: Regional Outlook And Key Takeaways
Fig. 37 Global Cardiac Safety Services market share and leading players
Fig. 38 North America, by country
Fig. 39 North America market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 40 US key country dynamics
Fig. 41 US market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 42 Canada key country dynamics
Fig. 43 Canada market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 44 Mexico key country dynamics
Fig. 45 Mexico market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 46 Europe market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 47 UK key country dynamics
Fig. 48 UK market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 49 Germany key country dynamics
Fig. 50 Germany market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 51 France key country dynamics
Fig. 52 France market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 53 Italy key country dynamics
Fig. 54 Italy market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 55 Spain key country dynamics
Fig. 56 Spain market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 57 Denmark key country dynamics
Fig. 58 Denmark market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 59 Sweden key country dynamics
Fig. 60 Sweden market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 61 Norway key country dynamics
Fig. 62 Norway market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 63 Asia Pacific market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 64 China key country dynamics
Fig. 65 China market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 66 Japan key country dynamics
Fig. 67 Japan market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 68 India key country dynamics
Fig. 69 India market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 70 Thailand key country dynamics
Fig. 71 Thailand market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 72 South Korea key country dynamics
Fig. 73 South Korea market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 74 Australia key country dynamics
Fig. 75 Australia market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 76 Latin America market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 77 Brazil key country dynamics
Fig. 78 Brazil market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 79 Argentina key country dynamics
Fig. 80 Argentina market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 81 Middle East and Africa market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 82 South Africa key country dynamics
Fig. 83 South Africa market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 84 Saudi Arabia key country dynamics
Fig. 85 Saudi Arabia market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 86 UAE key country dynamics
Fig. 87 UAE market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 88 Kuwait key country dynamics
Fig. 89 Kuwait market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 90 Market share of key market players - Cardiac Safety Services market
Companies Mentioned
- Medpace
- IQVIA
- PPD Inc.,
- Charles River Laboratories
- Wuxi AppTec
- Eurofins Scientific
- Celerion
- Nova Research Laboratories
- Laboratory Corporation of America Holdings
- Koninklije Philips N.V.
- ICON Plc.
- SGS S.A.
- Clario
- Certara
- Richmond Pharmacology
- Biotrial
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | January 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 0.97 Billion |
| Forecasted Market Value ( USD | $ 1.92 Billion |
| Compound Annual Growth Rate | 12.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |