Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, market growth confronts significant hurdles due to a complex and shifting regulatory environment that establishes high barriers to entry. Strict oversight concerning laboratory-developed tests has created substantial uncertainty and elevated compliance costs for manufacturers, potentially delaying the launch of innovative diagnostic assays. These regulatory pressures are often exacerbated by inconsistent reimbursement policies across various jurisdictions, which limit patient access to advanced genetic testing and constrain the revenue prospects for industry stakeholders.
Market Drivers
Rapid technological advancements in Next-Generation Sequencing are fundamentally transforming the DNA diagnostics sector by increasing throughput and shortening turnaround times. The deployment of high-capacity sequencing platforms allows laboratories to process genetic samples with exceptional speed and cost-effectiveness, enabling broader clinical adoption. For instance, Illumina's "Financial Results for Fourth Quarter and Fiscal Year 2023," released in February 2024, noted the shipment of 352 NovaSeq X instruments during the fiscal year, indicating robust market adoption of cutting-edge sequencing infrastructure. This technological proliferation facilitates the availability of comprehensive genomic profiling, permitting the detection of complex genetic variants that were previously challenging to identify, thereby accelerating the integration of genomics into routine medical practice.Simultaneously, the expanding application of diagnostics in oncology is a major growth engine, driven by the critical need for precision medicine and early disease detection. With the global cancer burden intensifying, healthcare providers are increasingly relying on liquid biopsies and genomic testing to tailor treatment plans. In 2024, the World Health Organization's International Agency for Research on Cancer estimated there were approximately 20 million new cancer cases globally in 2022, highlighting the immense scale of diagnostic requirements. Consequently, specialized providers are seeing a surge in demand for cancer-specific assays; illustrating this, Guardant Health reported in its February 2024 "Fourth Quarter and Full Year 2023 Financial Results" a 39% increase in clinical testing volume, delivering 172,900 tests to customers throughout the year.
Market Challenges
The intricate and evolving regulatory landscape stands as a major impediment to the growth of the Global DNA Diagnostics Market. The enforcement of stringent oversight, particularly regarding Laboratory-Developed Tests (LDTs) and in vitro diagnostics, has created substantial barriers to entry for clinical laboratories and manufacturers. These heightened regulatory requirements necessitate exhaustive validation procedures, compelling companies to divert significant resources toward compliance rather than innovation. This shift directly increases the capital required to introduce new assays and prolongs development timelines, effectively postponing the commercialization of advanced diagnostic tools and restricting patient access to essential genetic testing.The financial strain resulting from these compliance hurdles is both severe and quantifiable. According to MedTech Europe in 2024, the costs associated with certification and maintenance for diagnostic manufacturers under the new In Vitro Diagnostic Regulation have escalated by up to 100% compared to previous directives. This dramatic rise in operational expenditures discourages investment in new test development and forces companies to reconsider their strategic priorities, ultimately stifling the sector's potential for expansion and revenue growth.
Market Trends
Laboratories are increasingly embedding artificial intelligence and machine learning algorithms into bioinformatics workflows to automate data interpretation, enhance diagnostic accuracy, and manage the massive datasets produced by sequencing. This integration addresses the critical bottleneck of manual variant analysis, allowing for scalable clinical decision support and faster identification of complex genetic conditions. Validating this widespread adoption, SOPHiA GENETICS stated in its March 2025 "Fourth Quarter and Full Year 2024 Results" that the company conducted a record 352,000 genomic analyses using its data-driven platform in fiscal year 2024, representing an 11% year-over-year volume growth.Technological advancements are simultaneously enabling the decentralization of DNA testing, moving complex molecular diagnostics from centralized laboratories to portable, rapid testing devices suitable for clinics and remote settings. This shift dramatically reduces turnaround times from days to minutes, facilitating immediate treatment decisions for infectious diseases and acute conditions without the need for heavy infrastructure. Underscoring this market evolution, bioMérieux reported in its "2024 Financial Results" in March 2025 that sales of the SPOTFIRE rapid point-of-care molecular system reached nearly €95 million in 2024, with the installed base expanding by 2,200 units during the year.
Key Players Profiled in the DNA Diagnostics Market
- Illumina, Inc.
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- QIAGEN N.V.
- Abbott Laboratories
- Bio-Rad Laboratories, Inc.
- Hologic, Inc.
- Agilent Technologies, Inc.
- Siemens Healthineers AG
Report Scope
In this report, the Global DNA Diagnostics Market has been segmented into the following categories:DNA Diagnostics Market, by Technology:
- PCR-based Diagnostics
- NGS DNA Diagnosis
- In-Situ Hybridization Diagnostics
- Microarrays-based Diagnostics
- Other Technologies
DNA Diagnostics Market, by Application:
- Cancer Genetics Tests
- Infectious Diseases DNA Testing
- Newborn Genetic Screening
- Preimplantation & Reproductive Diagnosis
- Non-Infectious Diseases DNA Testing
- Newborn Genetic Screening
- Preimplantation & Reproductive Diagnosis
- Non-Infectious Diseases DNA Testing
DNA Diagnostics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global DNA Diagnostics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this DNA Diagnostics market report include:- Illumina, Inc.
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- QIAGEN N.V.
- Abbott Laboratories
- Bio-Rad Laboratories, Inc.
- Hologic, Inc.
- Agilent Technologies, Inc.
- Siemens Healthineers AG
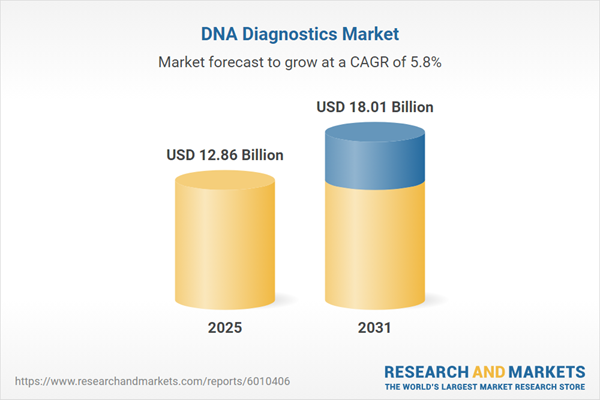
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
| Estimated Market Value ( USD | $ 12.86 Billion |
| Forecasted Market Value ( USD | $ 18.01 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |