Global Cardiac Troponin Market Analysis
The global cardiac troponin market is witnessing substantial growth due to advancements in medical technologies and increasing prevalence of cardiovascular diseases. Cardiac troponin is a critical biomarker for diagnosing heart attacks and other cardiac conditions, making it essential in emergency and routine diagnostic settings. The demand for accurate and early diagnosis of cardiac events is further driving market growth.Market Drivers
Advancements in Diagnostic Technologies: Continuous innovations in diagnostic tools and assays have significantly enhanced the sensitivity and specificity of cardiac troponin tests. These advancements are driving market growth as healthcare providers seek more reliable and faster diagnostic solutions for cardiac events.Rising Prevalence of Cardiovascular Diseases: The increasing incidence of heart diseases, such as acute coronary syndrome, myocardial infarction, and congestive heart failure, is fueling the demand for cardiac troponin tests. The aging population and lifestyle-related risk factors contribute to the growing number of cardiovascular cases, necessitating effective diagnostic tools.
Increased Demand for Precise Cardiac Health Monitoring: There is a growing emphasis on accurate and timely diagnosis of cardiac conditions to improve patient outcomes. Cardiac troponin tests provide crucial information for managing heart diseases, making them indispensable in clinical practice. The demand for precise cardiac health monitoring is driving the adoption of troponin assays.
Supportive Healthcare Investments: Government initiatives and investments in healthcare infrastructure are promoting the development and adoption of advanced diagnostic solutions. Supportive policies and financial incentives for innovation in cardiac diagnostics are contributing to market growth, ensuring better access to troponin tests.
Market Challenges
High Costs of Advanced Diagnostic Tools: The high cost associated with advanced cardiac troponin tests and instruments can limit accessibility, particularly in cost-sensitive healthcare environments. This financial barrier is a significant challenge for widespread adoption.Regulatory and Reimbursement Issues: Stringent regulatory requirements and reimbursement challenges can hinder market growth. Navigating complex approval processes and securing adequate reimbursement for advanced diagnostic tests can be time-consuming and costly for manufacturers and healthcare providers.
Limited Awareness in Developing Regions: The limited awareness and understanding of cardiac troponin tests in developing regions can hinder market growth. Increasing knowledge about the importance of early diagnosis and the availability of advanced diagnostic tools through educational initiatives is essential to promoting wider adoption.
Future Opportunities
Expansion into Emerging Markets: Expanding into emerging markets with improving healthcare infrastructure presents substantial growth opportunities. These regions offer untapped potential for increasing access to cardiac troponin tests and improving patient outcomes.Development of Novel Diagnostic Solutions: The development of novel diagnostic solutions, such as high-sensitivity troponin assays and point-of-care testing devices, can enhance the accuracy and convenience of cardiac diagnostics. Innovations in diagnostic technologies are expected to drive market growth by providing more efficient and reliable solutions.
Collaborations and Partnerships: Strategic partnerships between diagnostic companies, healthcare providers, and research institutions can drive market expansion. Collaborations can facilitate research, development, and distribution efforts, enhancing the availability and quality of cardiac troponin tests.
Integration with Advanced Technologies: Incorporating advanced technologies, such as artificial intelligence and machine learning, can enhance the functionality and efficiency of cardiac troponin tests. These innovations can drive market growth by providing more accurate and personalized diagnostic solutions.
Global Cardiac Troponin Market Trends
Rising Adoption of Point-of-Care Testing: The adoption of point-of-care testing (POCT) for cardiac biomarkers is increasing due to their effectiveness in providing rapid diagnostic results. POCT allows for immediate decision-making in emergency settings, which is crucial for acute cardiac events. This trend is driving market growth as more healthcare providers incorporate POCT into their diagnostic protocols, improving patient outcomes through timely interventions.Focus on High-Sensitivity Troponin Assays: There is a growing trend towards the use of high-sensitivity troponin assays in clinical practice. These assays offer improved diagnostic accuracy and early detection of cardiac events, which is vital for preventing complications and initiating early treatment. High-sensitivity assays are becoming the standard of care, enhancing patient care and supporting market growth by providing more reliable and actionable diagnostic information.
Increased Investment in R&D: Investment in research and development for cardiac troponin tests is on the rise. Companies are dedicating significant resources to discovering new diagnostic applications and improving existing tests, fostering innovation in the market. This includes developing more sensitive and specific assays, integrating advanced technologies like AI for better analysis, and exploring new biomarkers that can complement troponin testing, thereby expanding the scope of cardiac diagnostics.
Emphasis on Preventive Care: Ensuring preventive care in cardiac health is a top priority. Advances in diagnostic technologies are enabling early detection and intervention, reducing the incidence of severe cardiac events. Preventive care strategies, supported by regular monitoring and early diagnosis through advanced troponin tests, are driving market growth. This emphasis on preventive care is expected to continue driving innovation and adoption in cardiac diagnostics, ultimately improving long-term patient outcomes.
Global Cardiac Troponin Market Segmentation
Market Breakup by Product Type
- Instrument
- Kits and Reagents
Market Breakup by Type
- Troponin T
- Troponin I
Market Breakup by Application
- Acute Coronary Syndrome
- Myocardial Infarction
- Congestive Heart Failure
- Others
Market Breakup by End User
- Hospitals
- Diagnostic Centres
- Homecare Settings
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Cardiac Troponin Market Competitive Landscape
The global cardiac troponin market features several key players actively shaping the competitive landscape. Notable companies include Abbott Laboratories, F. Hoffmann-La Roche Ltd, Beckman Coulter, Inc., Creative Diagnostics, bioMérieux SA, Thermo Fisher Scientific Inc., Siemens Healthineers AG, Danaher Corporation, Randox Laboratories Ltd, and Eurolyser Diagnostica GmbH. These companies engage in activities such as mergers and acquisitions, research initiatives, product introductions, and strategic partnerships to expand their market presence and capabilities. These activities drive innovation and growth within the cardiac troponin market, ensuring continuous improvement and broadening service offerings.Key Questions Answered in the Report
- What is the projected CAGR for the global cardiac troponin market during the forecast period 2024-2032?
- How are advancements in diagnostic technologies influencing the growth of the cardiac troponin market?
- What factors are contributing to the increasing prevalence of cardiovascular diseases globally?
- How is the rising demand for precise cardiac health monitoring impacting the market for cardiac troponin tests?
- What role do government initiatives and healthcare investments play in driving the cardiac troponin market?
- What are the main challenges associated with the high costs of advanced cardiac troponin diagnostic tools?
- How do regulatory and reimbursement issues affect the adoption of cardiac troponin tests?
- What opportunities exist for expanding the cardiac troponin market into emerging regions?
- How is the development of novel diagnostic solutions expected to influence the market for cardiac troponin tests?
- In what ways are collaborations and partnerships shaping the growth and innovation in the cardiac troponin market?
- How does the rising adoption of point-of-care testing for cardiac biomarkers drive market growth?
- What impact does the emphasis on preventive care have on the adoption and innovation of cardiac troponin diagnostics?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the cardiac troponin market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the cardiac troponin market.
- The study maps the leading, as well as the fastest-growing, regional markets, enabling stakeholders to identify key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders analyze the level of competition within the cardiac troponin industry and its attractiveness.
- The competitive landscape section allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Siemens Healthcare GmbH
- Eurolyser Diagnostica GmbH
- Beckman Coulter, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2024 |
| Forecast Period | 2024 - 2032 |
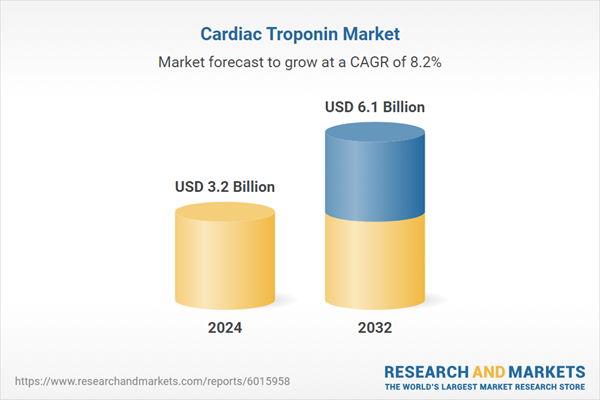
| Estimated Market Value ( USD | $ 3.2 Billion |
| Forecasted Market Value ( USD | $ 6.1 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |