Exocrine Pancreatic Insufficiency Treatment Market Overview
Exocrine pancreatic insufficiency (EPI) is a condition characterized by the inability of the pancreas to produce enough digestive enzymes to properly digest food. The treatment includes PERT, which involves taking pancreatic enzyme supplements with meals and snacks to replace the deficient enzymes. The enzyme supplements typically contain lipase, protease, and amylase to aid in the digestion of fats, proteins, and carbohydrates.EPI is primarily caused by conditions that affect pancreatic function, such as chronic pancreatitis, cystic fibrosis, pancreatic cancer, and pancreatic surgery. The prevalence of these underlying conditions contributes to the growing demand for EPI treatment market.
The pharmaceutical companies are focusing on developing advanced formulations of pancreatic enzymes to improve stability, efficacy, and patient compliance. These innovations aim to optimize treatment outcomes and enhance patient satisfaction, which is expected to drive the market growth.
Exocrine Pancreatic Insufficiency Treatment Market Growth Drivers
Increasing Incidence and Prevalence of Underlying Conditions
Chronic pancreatitis is a significant cause of EPI, which is increasingly diagnosed due to rising cases linked to alcohol abuse, obesity, and other factors. This genetic condition affects pancreatic function, leading to EPI in a substantial number of patients. According to an article published by NCB I, chronic pancreatitis is the most common pancreatic disease associated with EPI.Other conditions like pancreatic cancer and surgical interventions on the pancreas can impair its ability to produce digestive enzymes, necessitating EPI treatment. According to an article published by NCB I, pancreatic surgery alters digestive anatomy, the correct mixing of food, bile, and pancreatic enzymes and reduces the pancreatic volume. Different procedures are associated with different degrees of EPI. The growing number of individuals diagnosed with chronic pancreatitis directly expands the pool of patients at risk of developing EPI, thereby increasing the demand for its treatment market.
Technological Advancements in Treatment Options is Expected to Propel Exocrine Pancreatic Insufficiency Treatment Market Demand
The technological advancements in treatment options play a crucial role in driving the growth and development of the treatment market. The technological innovations enable the development of more effective formulations of pancreatic enzymes, including lipase, protease, and amylase. These formulations are designed to better mimic natural pancreatic enzyme activity, thereby improving digestion and nutrient absorption in patients with EPI. Pancreatic Enzyme Replacement Therapy (PERT) remains the mainstay of EPI treatment, with ongoing advancements in enzyme formulations, delivery systems, and dosing regimens to enhance efficacy and patient compliance.
The advancement in treatment includes other alternative therapies, exploration of adjunctive therapies, such as proton pump inhibitors (PPIs), dietary modifications, and nutritional supplements, provides additional treatment options tailored to patient needs. This innovation leads to increase in market demand.
Exocrine Pancreatic Insufficiency Treatment Market Trends
The market is witnessing several trends and developments to improve the current global scenario. Some of the notable trends are as follows:Advancements in Enzyme Replacement Therapy
There is ongoing research and development aimed at enhancing the efficacy, stability, and bioavailability of pancreatic enzyme replacement therapy (PERT). The innovations include formulations with optimized enzyme ratios, enteric-coated tablets for improved delivery, and microencapsulation technologies to protect enzymes from degradation. These advancements are expected to drive the market growth.Exploration of Novel Therapies
The ongoing research efforts are focused on exploring novel therapeutic approaches beyond traditional enzyme replacement, including enzyme enhancers, pancreatic duct clearance agents, and targeted therapies addressing underlying disease mechanisms.Personalized Medicine Approach
The increasing use of genetic testing helps identify specific mutations (e.g., CFTR mutations in cystic fibrosis-related EPI) that influence treatment responses. This allows for personalized treatment plans tailored to individual genetic profiles, optimizing therapy effectiveness and patient outcomes. This is expected to drive market growth.Exocrine Pancreatic Insufficiency Treatment Market Segmentation
“Exocrine Pancreatic Insufficiency Treatment Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Treatment
- Pancreatic Enzyme Replacement Therapy (PERT)
- Nutritional Therapy (Dietary Supplements)
- Others
Market Breakup by Indication
- Abdominal Pain
- Constipation
- Diarrhea
- Fatty Stools
- Weight Loss
- Others
Market Breakup by End User
- Hospitals
- Homecare
- Specialty Centers
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Exocrine Pancreatic Insufficiency Treatment Market Share
Market Segmentation Based on Treatment is Anticipated to Witness Substantial Growth
By treatment, the market is segmented into pancreatic enzyme replacement therapy (PERT), nutritional therapy (dietary supplements) and others. The pancreatic enzyme replacement therapy (PERT) segment is expected to dominate the market because it is the most recommended treatment. It helps in managing EPI symptoms by replacing the deficient pancreatic enzymes (lipase, protease, amylase) required for proper digestion of fats, proteins, and carbohydrates.Exocrine Pancreatic Insufficiency Treatment Market Analysis by Region
Regionally, the market report offers an insight into the United States, EU-4 and the United Kingdom, Germany, France, Italy, Spain, United Kingdom, Japan, and India. The United States, having a robust healthcare infrastructure and a strong presence of prominent pharmaceutical companies, is estimated to hold a high market value. The robust research and development activities in pharmaceutical and biotechnology sectors, leading to continuous advancements in pancreatic enzyme replacement therapy (PERT) formulations, driving the regional demand.Germany holds a high exocrine pancreatic insufficiency treatment market value as the region boasts a well-developed healthcare system with robust diagnostic capabilities and access to specialized care, facilitating early detection and management of EPI.
Additionally, Japan is expected to witness substantial market growth as the region has made significant progress in raising awareness among healthcare providers about gastrointestinal disorders, including EPI. This has led to improved diagnosis and management of the condition and thus driving the market demand.
Leading Players in the Exocrine Pancreatic Insufficiency Treatment Market
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, fundings and investment analysis, and strategic initiatives by the leading key players. The major companies in the market are as follows:AbbVie Inc.
AbbVie Inc., a prominent pharmaceutical company, has been involved in the treatment of exocrine pancreatic insufficiency (EPI) through its development and marketing of pancreatic enzyme replacement therapy (PERT) products.Johnson & Johnson Services, Inc.
Johnson & Johnson Services, Inc., as a global pharmaceutical company focuses on several therapeutic areas such as pharmaceuticals, generics, eye care, and oncology, among others, including EPI.Other key players in the market include Nordmark Arzneimittel GmbH and Co. KG, Digestive Care, Inc., Cilian AG, Chiesi Farmaceutici S.p.A., Anthera Pharmaceuticals, Inc., and Aptalis Pharma Inc. among others.
Key Questions Answered in the Exocrine Pancreatic Insufficiency Treatment Market Report
- What was the exocrine pancreatic insufficiency treatment market value in 2023?
- What is the exocrine pancreatic insufficiency treatment market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on treatment?
- What is market segmentation based on indication?
- What is market segmentation based on end user?
- What are the major factors aiding the exocrine pancreatic insufficiency treatment market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major trends influencing the market?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- How does the growing cases of chronic pancreatis affect the market landscape?
- How does the clinical trials impact the market size?
- Who are the key players involved in the exocrine pancreatic insufficiency treatment market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- AbbVie Inc.
- Johnson & Johnson Services, Inc.
- Chiesi Farmaceutici S.p.A.
- Nordmark Arzneimittel GmbH and Co. KG
- Digestive Care, Inc.
- Cilian AG
- Anthera Pharmaceuticals, Inc.
- Aptalis Pharma Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
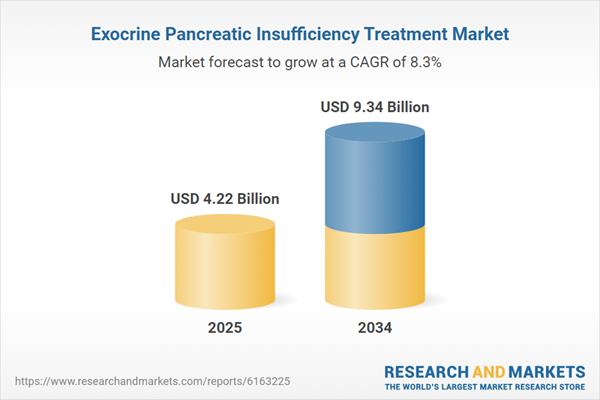
| Estimated Market Value ( USD | $ 4.22 Billion |
| Forecasted Market Value ( USD | $ 9.34 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |