Global Rare Haematology Disorders Market Analysis
The global rare haematology disorders market encompasses a diverse group of blood-related diseases that affect a small percentage of the population. These disorders include conditions such as haemophilia, sickle cell anaemia, thalassaemia, and other rare blood clotting and bleeding disorders. The market has seen significant growth in recent years, driven by increased awareness, advancements in diagnostic technologies, and the development of innovative therapies. Despite the rarity of these conditions, they pose significant health challenges for patients, making the demand for effective treatments and management strategies crucial.Market Drivers
Several factors are driving the growth of the global rare haematology disorders market. One of the primary drivers is the increasing prevalence of rare blood disorders due to improved diagnostic capabilities. Early and accurate diagnosis has led to better disease management and an increased focus on treatment development. Additionally, technological advancements in gene therapy and targeted therapies are transforming the treatment landscape, offering hope for more effective and personalised treatments for patients with rare haematology disorders.Government initiatives and funding aimed at rare disease research have also played a crucial role in market growth. Programmes that incentivise the development of orphan drugs - medications designed specifically for rare diseases - have encouraged pharmaceutical companies to invest in this market. Moreover, the growing patient advocacy and support networks are raising awareness and driving demand for better treatment options, leading to increased market expansion.
Market Challenges
Despite the positive growth trajectory, the global rare haematology disorders market faces several challenges. One of the major hurdles is the high cost of treatment. Many therapies, especially gene and enzyme replacement therapies, are expensive, making them inaccessible to a significant portion of the population, particularly in low- and middle-income countries. Additionally, the complex regulatory landscape for the approval of orphan drugs can delay the introduction of new treatments, hindering market growth.Another challenge is the limited patient population, which makes it difficult to conduct large-scale clinical trials and gather sufficient data for the development of new therapies. This limitation often results in a lack of comprehensive treatment options and a slow pace of innovation in the field. Furthermore, awareness of rare haematology disorders remains low in certain regions, leading to delayed diagnosis and treatment.
Future Opportunities
The future of the global rare haematology disorders market looks promising, with several opportunities for growth and innovation. Emerging markets offer significant potential as healthcare infrastructure improves and awareness of rare disorders increases. As governments in these regions allocate more resources to healthcare, the accessibility of advanced treatments is expected to rise.The development of gene editing technologies, such as CRISPR-Cas9, presents a groundbreaking opportunity for curing or significantly mitigating the effects of rare haematology disorders. These technologies have the potential to offer long-term solutions and even permanent cures, revolutionising the treatment landscape.
Collaborative research efforts between academic institutions, pharmaceutical companies, and patient advocacy groups are likely to accelerate the development of new therapies. These partnerships can lead to more efficient drug development processes and bring innovative treatments to market faster.
Lastly, digital health solutions and telemedicine offer new ways to manage rare haematology disorders, particularly in remote areas. These technologies can improve patient monitoring, enhance access to specialist care, and support the ongoing management of these complex conditions.
Global Rare Haematology Disorders Market Trends
The global rare haematology disorders market is evolving rapidly, influenced by technological advancements, increased awareness, and a growing emphasis on personalised medicine. As the landscape shifts, several key trends are shaping the future of this specialised market.1. Gene and Cell Therapies Leading the Way: The rise of gene and cell therapies is a significant trend in the rare haematology disorders market. These cutting-edge treatments offer the potential to address the root cause of many rare blood disorders by correcting genetic abnormalities or replacing defective cells. The success of therapies like gene editing and CAR-T cell treatments is paving the way for more personalised and long-lasting solutions, which are expected to become the standard of care in the coming years.
2. Expansion of Orphan Drug Designations: The increasing number of orphan drug designations is driving innovation in the market. Regulatory incentives, including market exclusivity and tax benefits, are encouraging pharmaceutical companies to invest in the development of treatments for rare haematology disorders. This trend is leading to a more robust pipeline of therapies, with several promising drugs expected to receive approval in the near future.
3. Growth in Patient Advocacy and Support Networks: Patient advocacy groups are playing a crucial role in the rare haematology disorders market by raising awareness, influencing policy, and driving research initiatives. These organisations are increasingly partnering with pharmaceutical companies and research institutions to accelerate the development of new treatments and improve patient access to care. This growing collaboration is helping to bridge the gap between patients and treatment providers, leading to better outcomes and more comprehensive care.
4. Adoption of Advanced Diagnostic Technologies: Advances in diagnostic technologies, such as next-generation sequencing and molecular diagnostics, are transforming the way rare haematology disorders are identified and managed. Early and accurate diagnosis is becoming more achievable, enabling healthcare providers to implement targeted treatment plans more quickly. This trend is expected to reduce the burden of misdiagnosis and improve the overall prognosis for patients with rare blood disorders.
5. Increased Focus on Precision Medicine: Precision medicine is gaining traction in the rare haematology disorders market, with treatments increasingly tailored to the specific genetic and molecular profiles of patients. This approach is leading to more effective therapies with fewer side effects, as well as a shift towards personalised treatment strategies that cater to individual patient needs. As precision medicine continues to advance, it is expected to become a cornerstone of care for patients with rare haematology disorders.
Global Rare Haematology Disorders Market Segmentation
Market Breakup by Treatment Type
- Plasma Derived
- Recombinant
Market Breakup by Disease Type
- Langerhans Cell Histiocytosis (LCH)
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Gaucher Disease
- Polycythemia Vera
- Others
Market Breakup by Dosage Form
- Oral
- Injectable
- Others
Market Breakup by Age Group
- Adult (18+)
- Pediatric (0-17)
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Rare Haematology Disorders Market Competitive Landscape
The competitive landscape of the global rare haematology disorders market is characterised by the presence of several key players, including Bristol-Myers Squibb Company, Novo Nordisk, F. Hoffmann-La Roche Ltd, Emmaus Medical, Inc., Pfizer Inc., Octapharma, Novartis, Grifols, CSL Behring, and Takeda. These companies are actively engaged in market-shaping activities such as mergers and acquisitions, research and development initiatives, new product introductions, and strategic partnerships. These activities aim to expand their product portfolios, enhance market presence, and drive innovation in the treatment of rare haematology disorders. The competitive environment is dynamic, with companies striving to gain a competitive edge through advancements in gene therapy, biologics, and orphan drug development, positioning them as leaders in this specialised market.Key Questions Answered in the Report
- What is the current and future performance of the global rare haematology disorders market?
- What are the main challenges facing the global rare haematology disorders market?
- What are the key drivers of the global rare haematology disorders market?
- What emerging trends are shaping the future of the global rare haematology disorders market?
- How are advances in diagnostic technologies improving the early detection of rare haematology disorders?
- How is precision medicine transforming treatment strategies in the rare haematology disorders market?
- What factors are driving the rapid growth of the recombinant segment in the rare haematology disorders market?
- Which disease types are gaining attention in the rare haematology disorders market, and why?
- Why does the adult segment dominate the rare haematology disorders market?
- What are the common strategies used by key players in the global rare haematology disorders market?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the rare haematology disorders market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the rare haematology disorders market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the rare haematology disorders industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Bristol-Myers Squibb Company
- Novo Nordisk A/S
- F. Hoffmann-La Roche Ltd
- Emmaus Medical, Inc.
- Pfizer Inc.
- Bayer AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2024 |
| Forecast Period | 2024 - 2032 |
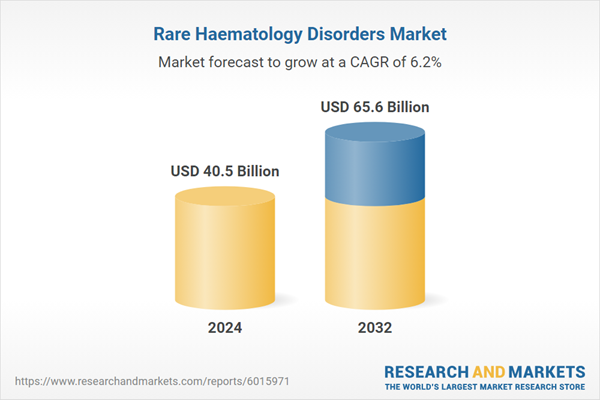
| Estimated Market Value ( USD | $ 40.5 Billion |
| Forecasted Market Value ( USD | $ 65.6 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 6 |