Retinitis Pigmentosa Treatment Market Analysis
Retinitis pigmentosa (RP) is a group of inherited retinal disorders characterised by progressive peripheral vision loss and night vision difficulties, eventually leading to central vision impairment. As a degenerative condition, RP affects the photoreceptor cells in the retina, leading to gradual vision loss. The global market for retinitis pigmentosa treatment is driven by advancements in gene therapy, retinal implants, and pharmacological treatments aimed at slowing disease progression or restoring some degree of vision. With an increasing prevalence of RP and ongoing research in ophthalmology, the market for RP treatments is expected to grow steadily in the coming years.Market Drivers
The primary drivers of the retinitis pigmentosa treatment market include the rising incidence of retinal diseases, advancements in medical technology, and increased funding for ophthalmic research. As gene therapy emerges as a promising avenue for treating genetic disorders like RP, significant investments are being made to develop and commercialise these therapies. Additionally, the growing awareness of retinal diseases and the availability of better diagnostic tools are driving early diagnosis and treatment, further fuelling market growth. The ageing population, particularly in developed regions, also contributes to the rising demand for RP treatments, as the prevalence of retinal disorders increases with age.Challenges
Despite the promising outlook, the retinitis pigmentosa treatment market faces several challenges. One of the primary obstacles is the high cost associated with advanced treatments, such as gene therapy and retinal implants, which limits access for many patients. Moreover, the complexity of the disease, with its numerous genetic mutations, poses significant challenges for developing a one-size-fits-all treatment. Regulatory hurdles also present a barrier, as novel therapies require rigorous testing and approval processes, which can delay market entry. Additionally, the lack of curative treatments means that current options focus on managing symptoms rather than offering a definitive cure, which can impact patient satisfaction and market penetration.Future Opportunities
The future of the retinitis pigmentosa treatment market holds significant potential, driven by ongoing research and development in gene therapy, stem cell therapy, and retinal implants. As personalised medicine continues to evolve, targeted therapies based on individual genetic profiles could offer more effective treatment options for RP patients. The increasing adoption of artificial intelligence in ophthalmology may also lead to earlier diagnosis and better management of the disease. Collaborations between biotech companies, research institutions, and healthcare providers are expected to accelerate the development and accessibility of new treatments. Moreover, expanding healthcare access in emerging markets presents an untapped opportunity for market growth, as awareness and diagnosis of retinal diseases improve globally.Retinitis Pigmentosa Treatment Market Trends
The retinitis pigmentosa treatment market is witnessing significant developments, driven by advancements in medical technology and increasing research into genetic therapies. As the global prevalence of retinal disorders rises, the market is evolving with new trends that are reshaping the landscape of treatment options.Key Market Trends
- Emergence of Gene Therapy: Gene therapy is becoming a pivotal trend in the retinitis pigmentosa treatment market. Companies are developing gene-specific therapies aimed at correcting the genetic mutations responsible for RP. With the approval of treatments like Luxturna, there is growing confidence in the potential of gene therapy to offer long-term solutions for retinal degeneration, leading to increased investment and research in this area.
- Advancements in Retinal Implants: Retinal implants, such as the Argus II Retinal Prosthesis System, are gaining traction as a treatment option for advanced retinitis pigmentosa. These devices, which can partially restore vision by converting visual information into electrical impulses, are becoming more sophisticated, with improved resolution and functionality. As technology continues to advance, the demand for these implants is expected to grow, particularly among patients with severe vision loss.
- Personalised Medicine: The shift towards personalised medicine is another significant trend in the RP treatment market. With the advent of genetic testing, treatments can be tailored to the specific genetic profile of patients. This approach not only enhances the effectiveness of therapies but also reduces the risk of adverse effects. Personalised medicine is likely to play an increasingly important role in managing retinitis pigmentosa, as more targeted therapies are developed.
- Increased Focus on Early Diagnosis: Early diagnosis of retinitis pigmentosa is becoming more feasible with advancements in imaging technologies and genetic screening. Early intervention can slow the progression of the disease, and healthcare providers are placing greater emphasis on early detection. This trend is likely to drive demand for diagnostic tools and contribute to better patient outcomes.
- Expanding Research in Stem Cell Therapy: Stem cell therapy is emerging as a promising area of research in the treatment of retinitis pigmentosa. Scientists are exploring the potential of stem cells to regenerate damaged retinal cells and restore vision. While still in the experimental stages, stem cell therapy holds considerable promise, and continued research in this area is expected to lead to new treatment options in the future.
Retinitis Pigmentosa Treatment Market Segmentation
Market Breakup by Type
- Autosomal Recessive RP
- Autosomal Dominant RP
- X-Linked RP
Market Breakup by Treatment
- Drug
- Vitamin A Palmitate
- Acetazolamide
- Others
- Device
- Sunglasses
- Implants
- Others
- Surgery
- Retinal Transplantation
- Corneal Neurotization
- Others
Market Breakup by Distribution Channel
- Hospital Retailer
- Retail Retailer
- Online
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Retinitis Pigmentosa Treatment Market Competitive Landscape
The competitive landscape of the retinitis pigmentosa treatment market is marked by significant activities, including mergers and acquisitions, research initiatives, product introductions, and strategic partnerships. Key players include ReNeuron Group plc, Ocugen, Inc., MeiraGTx Limited, Bausch Health Companies Inc., Pfizer Inc., Teva Pharmaceuticals, Novartis AG, and Astellas Pharma Inc. These companies are actively involved in developing innovative therapies, particularly in gene therapy and advanced drug formulations. Collaborative research and partnerships are common as firms seek to leverage expertise and expand their product pipelines. Mergers and acquisitions also play a crucial role in consolidating market positions and enhancing technological capabilities.Key Questions Answered in the Report
- What is the current and future performance of the retinitis pigmentosa treatment market?
- What are the main challenges facing the retinitis pigmentosa treatment market?
- What are the key drivers of the retinitis pigmentosa treatment market?
- What emerging trends are shaping the future of the retinitis pigmentosa treatment market?
- What are the future opportunities in the retinitis pigmentosa treatment market driven by personalised medicine?
- How are advancements in retinal implant technology influencing treatment options for advanced retinitis pigmentosa?
- Why is the Autosomal Dominant RP segment projected to experience significant growth in the retinitis pigmentosa treatment market?
- How do Vitamin A Palmitate and Acetazolamide play a crucial role in managing retinitis pigmentosa?
- What are the common strategies used by key players in the retinitis pigmentosa treatment market?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the rare haematology disorders market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the rare haematology disorders market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the rare haematology disorders industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Ionis Pharmaceuticals, Inc.
- Orphagen Pharmaceuticals, Inc.
- ReNeuron Group plc
- Ocugen, Inc.
- MeiraGTx Limited.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2024 |
| Forecast Period | 2024 - 2032 |
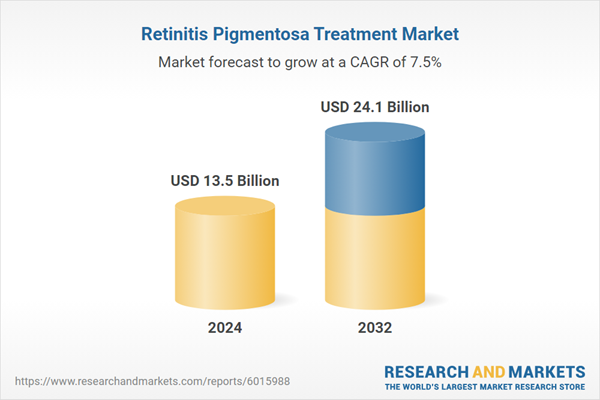
| Estimated Market Value ( USD | $ 13.5 Billion |
| Forecasted Market Value ( USD | $ 24.1 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |